Effect of Cortexin administration on Kisspeptin and Spexin expression after testicular torsion
Abstract
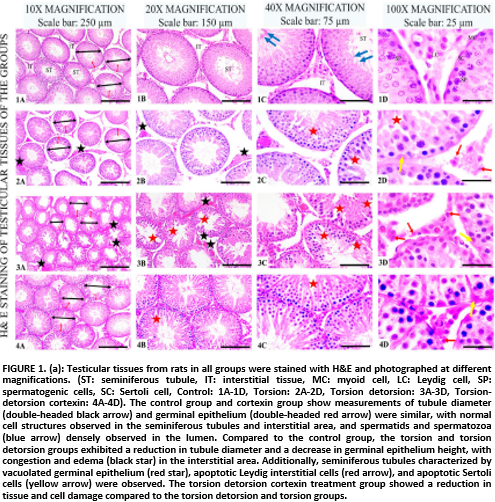
Kisspeptin–1 (KISS–1) and Spexin (SPX) are neuropeptides that play crucial roles in metabolism and sexual function, with their expression levels in tissues potentially influenced by antioxidant treatments. This study aimed to investigate the effects of cortexin treatment against ischemia–reperfusion injury (I/R) resulting from testicular torsion on KISS–1 and SPX levels in testicular tissues. Twenty–eight male Sprague–Dawley, rats, aged 8–10 weeks, were divided into four equal groups: control, torsion, torsion/ detorsion, and torsion/detorsion+cortexin. At the conclusion of the experiment, histopathological and immunohistochemical analyses were performed to assess the expressions of KISS–1, SPX, tumor necrosis factor–alpha (TNF–α), and Caspase–3 in the testicular tissues. For biochemical analyses, total antioxidant status (TAS) and total oxidant status (TOS) levels were measured in serum samples using the ELISA method, while malondialdehyde (MDA) levels were assessed spectrophotometrically in testicular tissues. The results showed that compared to the control group, the torsion and torsion/detorsion groups exhibited significant histopathological damage, along with increased levels of MDA, TOS, Caspase–3, and TNF–α, and decreased levels of TAS, KISS–1, and SPX in the testicular tissues. Conversely, in the torsion+detorsion+cortexin group, which received treatment for reperfusion injury, there was a notable reduction in tissue damage, with decreased levels of MDA, TOS, caspase–3, and TNF–α, alongside increased levels of TAS, KISS, and SPX. Cortexin decreases testicular damage by reducing oxidative stress, increases spermatogenesis by improving seminiferous tubule and germinal epithelial thickness, and regulates KISS–1 and SPX expression, which have effects on the reproductive system.
Downloads
References
Shamsi–Gamchi N, Razi M, Behfar M. Testicular torsion and reperfusion: evidences for biochemical and molecular alterations. Cell Stress Chaperones [Internet]. 2018; 23(3):429-439. doi: https://doi.org/gddx9f DOI: https://doi.org/10.1007/s12192-017-0855-0
Shimizu S, Tsounapi P, Dimitriadis F, Higashi Y, Shimizu T, Saito M. Testicular torsion–detorsion and potential therapeutic treatments: A possible role for ischemic postconditioning. Int. J. Urol. [Internet]. 2016; 23(6):454-463. doi: https://doi.org/f8qpt2 DOI: https://doi.org/10.1111/iju.13110
Wilhelm–Filho D, Torres MA, Bordin AL, Crezcynski–Pasa TB, Boveris A. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia–reperfusion injury. Mol. Aspects Med. [Internet]. 2004; 25(1-2):199-210. doi: https://doi.org/bhsgb4 DOI: https://doi.org/10.1016/j.mam.2004.02.020
Abdelzaher WY, Mostafa–Hedeab G, Sayed AboBakr Ali AH, Fawzy MA, Ahmed AF, Bahaa El–Deen MA, Welson NN, Aly Labib DA. Idebenone regulates sirt1/Nrf2/TNF–α pathway with inhibition of oxidative stress, inflammation, and apoptosis in testicular torsion/detorsion in juvenile rats. Hum. Exp. Toxicol. [Internet]. 2022; 41:9603271221102515. doi: https://doi.org/ph2v DOI: https://doi.org/10.1177/09603271221102515
Afolabi O, Alabi B, Omobowale T, Oluranti O, Iwalewa O. Cysteamine mitigates torsion/detorsion–induced reperfusion injury via inhibition of apoptosis, oxidative stress and inflammatory responses in experimental rat model. Andrologia [Internet]. 2022; 54(1):e14243. doi: https://doi.org/ph2w DOI: https://doi.org/10.1111/and.14243
Mohamed MZ, Morsy MA, Mohamed HH, Hafez HM. Paeonol protects against testicular ischaemia–reperfusion injury in rats through inhibition of oxidative stress and inflammation. Andrologia [Internet]. 2020; 52(6):e13599. doi: https://doi.org/ph2x DOI: https://doi.org/10.1111/and.13599
Stepanichev MY, Onufriev M, Peregud D, Lazareva N, Moiseeva YV, Nesterenko A, Novikova MR, Stefanova NA, Kolosova NG, Gulyaeva NV. The effects of cortexin on free–radical oxidation and inflammatory processes in rats with normal and accelerated aging. Neurochem. J. [Internet]. 2018; 12:184-194. doi: https://doi.org/ph2z DOI: https://doi.org/10.1134/S1819712418020113
Coulter PM, Bautista EA, Margulies JE, Watson JB. Identification of cortexin: A novel, neuron–specific, 82–residue membrane protein enriched in rodent cerebral cortex. J. Neurochem. [Internet]. 1993 ;61(2):756-759. doi: https://doi.org/d3php7 DOI: https://doi.org/10.1111/j.1471-4159.1993.tb02183.x
Mashin V, Belova L, Chaplanova O, Khusnullina A, Manasian A. Открытое клиническое исследование препарата кортексин при дисциркуляторной энцефалопатии [An open clinical trial of cortexin in treatment of brain ischemia]. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova [Internet]. 2014 [cited 22 Jan. 2024]; 114(9):49-52. Russian. Available in: https://goo.su/zgvatF
Eroğlu O, Karlıdağ T, Kuloğlu T, Keleş E, Kaygusuz İ, Yalçın Ş. The protective effect of cortexin on cisplatin–induced ototoxicity. J. Int. Adv. Otol. [Internet]. 2018; 14(1):27-33. doi: https://doi.org/ph22
Zarubina I, Shabanov P. Cortexin and cortagen as correcting agents in functional and metabolic disorders in the brain in chronic ischemia. Eksp. Klin. Farmakol. [Internet]. 2011 [cited 11 Jan. 2024]; 74(2):8-15. Available in: https://goo.su/NSjEk2
Kim DK, Yun S, Son GH, Hwang JI, Park CR, Kim JI, Kim K, Vaudry H, Seong JY. Coevolution of the spexin/galanin/ kisspeptin family: Spexin activates galanin receptor type II and III. Endocrinology [Internet]. 2014; 155(5):1864-1873. doi: https://doi.org/f6h475 DOI: https://doi.org/10.1210/en.2013-2106
Tng EL. Kisspeptin signalling and its roles in humans. Singapore Med. J. [Internet]. 2015; 56(12):649-656. doi: https://doi.org/f773g7 DOI: https://doi.org/10.11622/smedj.2015183
Xie Q, Kang Y, Zhang C, Xie Y, Wang C, Liu J, Yu C, Zhao H, Huang D. The role of kisspeptin in the control of the hypothalamic– pituitary–gonadal axis and reproduction. Front. Endocrinol. [Internet]. 2022; 13:925206. doi: https://doi.org/gqwbp4 DOI: https://doi.org/10.3389/fendo.2022.925206
Lv SY, Zhou YC, Zhang XM, Chen WD, Wang YD. Emerging roles of NPQ/spexin in physiology and pathology. Front. Pharmacol. [Internet]. 2019; 10:457. doi: https://doi.org/ph23 DOI: https://doi.org/10.3389/fphar.2019.00457
Ma A, Bai J, He M, Wong AO. Spexin as a neuroendocrine signal with emerging functions. Gen. Comp. Endocrinol. [Internet]. 2018; 265:90-96. doi: https://doi.org/gd6q4q DOI: https://doi.org/10.1016/j.ygcen.2018.01.015
Cohen Y, Hausken K, Bonfil Y, Gutnick M, Levavi–Sivan B. Spexin and a novel cichlid–specific spexin paralog both inhibit FSH and LH through a specific galanin receptor (Galr2b) in tilapia. Front. Endocrinol. [Internet]. 2020; 11:71. doi: https://doi.org/gsvqnf DOI: https://doi.org/10.3389/fendo.2020.00071
Porzionato A, Rucinski M, Macchi V, Stecco C, Malendowicz LK, De Caro R. Spexin expression in normal rat tissues. J. Histochem. Cytochem. [Internet]. 2010; 58(9):825-837. doi: https://doi.org/c78wmx DOI: https://doi.org/10.1369/jhc.2010.956300
Yazihan N, Ataoglu H, Koku N, Erdemli E, Sargin AK. Protective role of erythropoietin during testicular torsion of the rats. World J. Urol. [Internet]. 2007; 25:531-536. doi: https://doi.org/c7hnkr DOI: https://doi.org/10.1007/s00345-007-0200-9
Xia Z, Hu J, Han L, Xia Q, Shao F, Lin X. Effect of unilateral testicular torsion on contralateral testis in a rat model. Pediat. Sur Int. [Internet]. 2020; 36:529-536. doi: https://doi.org/ph24 DOI: https://doi.org/10.1007/s00383-020-04626-y
Bal R, Türk G, Tuzcu M, Yilmaz O, Ozercan I, Kuloglu T, Gür S, Nedzvetsky VS, Tykhomyrov AA, Andrievsky GV, Baydas G, Naziroglu M. Protective effects of nanostructures of hydrated C60 fullerene on reproductive function in streptozotocin– diabetic male rats. Toxicology [Internet]. 2011; 282(3):69-81. doi: https://doi.org/bxjt56 DOI: https://doi.org/10.1016/j.tox.2010.12.003
Oliveira Filho AB, Souza RSd, Azeredo–Oliveira MTVd, Peruquetti RL, Cedenho AP. Microdissection testicular sperm extraction causes spermatogenic alterations in the contralateral testis. Genet. Mol. Res. [Internet]. 2010; 9(3):1405-1413. doi: https://doi.org/bjxthr DOI: https://doi.org/10.4238/vol9-3gmr860
Kaplan S, Kırıcı P, Türk A. The effects of adalimumab on the rat autotransplantation endometriosis model: A placebo– controlled randomized study. Adv. Clin. Exp. Med. [Internet]. 2022; 31(4):417-426. doi: https://doi.org/gqwqrq DOI: https://doi.org/10.17219/acem/144369
Turk A, Ulas M, Karadag A, Kocaman N, Onalan E, Kuloglu T. The effects of N–acetylcysteine on transient receptor potential melastatin 2 channels activation and expression in testicular tissue of diabetic rats. Cureus [Internet]. 2023; 15(5):e38661 doi: https://doi.org/ph25 DOI: https://doi.org/10.7759/cureus.38661
Arena S, Iacona R, Antonuccio P, Russo T, Salvo V, Gitto E, Impellizzeri P, Romeo C. Medical perspective in testicular ischemia-reperfusion injury. Exp. Ther. Med. [Internet]. 2017; 13(5):2115-2122. doi: https://doi.org/f99dd5 DOI: https://doi.org/10.3892/etm.2017.4289
Moradi–Ozarlou M, Javanmardi S, Tayefi Nasrabadi H. Antioxidant property of Plantago major leaf extracts reduces testicular torsion/detorsion–induced ischemia/reperfusion injury in rats. Vet. Res. Forum. [Internet]. 2020; 11(1):27-33. doi: https://doi.org/ph26
Oral A, Halici Z, Bayir Y, Topcu A, Un H, Bilgin AO, Atmaca HT. Effects of oral zinc administration on long–term ipsilateral and contralateral testes damage after experimental testis ischaemia–reperfusion. Andrologia [Internet]. 2017; 49(6):e12673. doi: https://doi.org/ph27 DOI: https://doi.org/10.1111/and.12673
Tunçcan T, Yalçın Ş, Demir CF, Akın MM, Karlıdağ T, Keleş E, Kaygusuz İ. Efficacy of cortexin and methylprednisolone on traumatic facial nerve paralysis. J. Int. Adv. Otol. [Internet]. 2016; 12(3):303-309. doi: https://doi.org/ph28 DOI: https://doi.org/10.5152/iao.2016.1166
Eroğlu O, Karlıdağ T, Kuloğlu T, Keleş E, Kaygusuz İ, Yalçın Ş. The protective effect of cortexin on cisplatin–ınduced ototoxicity. J. Int. Adv. Otol. [Internet]. 2018; 14(1), 27-33. doi: https://doi.org/ph22 DOI: https://doi.org/10.5152/iao.2017.3825
Kurkin DV, Bakulin DA, Morkovin EI, Kalatanova AV, Makarenko IE, Dorotenko AR, Kovalev NS, Dubrovina MA, Verkholyak DV, Abrosimova EE, Smirnov AV, Shmidt MV, Tyurenkov IN. Neuroprotective action of cortexin, cerebrolysin and actovegin in acute or chronic brain ischemia in rats. PLoS One [Internet]. 2021; 16(7):e0254493. doi: https://doi.org/ph29 DOI: https://doi.org/10.1371/journal.pone.0254493
Gökçe A, Oktar S, Koc A, Gonenci R, Yalcinkaya F, Yonden Z, Duru M. Protective effect of thymoquinone in experimental testicular torsion. Urol. Int. [Internet]. 2010; 85(4):461-465. doi: https://doi.org/bjvfqr DOI: https://doi.org/10.1159/000318890
Abdullah DM, Alsemeh AE, Khamis T. Semaglutide early intervention attenuated testicular dysfunction by targeting the GLP–1–PPAR–α–Kisspeptin–Steroidogenesis signaling pathway in a testicular ischemia–reperfusion rat model. Peptides [Internet]. 2022; 149:170711. doi: https://doi.org/ph3b DOI: https://doi.org/10.1016/j.peptides.2021.170711
Fathy MA, Alsemeh AE, Habib MA, Abdel–Nour HM, Hendawy DM, Eltaweel AM, Abdelkhalek A, Ahmed MM, Desouky MK, Hua J, Fericean LM, Banatean–Dunea I, Arisha AH, Khamis T. Liraglutide ameliorates diabetic–induced testicular dysfunction in male rats: role of GLP–1/Kiss1/GnRH and TGF–β/Smadsignalingpathways.Front.Pharmacol.[Internet]. 2023; 14:1224985. doi: https://doi.org/ph3c DOI: https://doi.org/10.3389/fphar.2023.1224985
Liu Y, Sun L, Zheng L, Su M, Liu H, Wei Y, Li D, Wang Y, Dai C, Gong Y, Zhao C, Li Y. Spexin protects cardiomyocytes from hypoxia–induced metabolic and mitochondrial dysfunction. Naunyn–Schmiedebergs Arch. Pharmacol. [Internet]. 2020; 393:25-33. doi: https://doi.org/ph3d DOI: https://doi.org/10.1007/s00210-019-01708-0
Yazgan B, Avcı F, Memi G, Tastekin E. Inflammatory response and matrix metalloproteinases in chronic kidney failure: Modulation by adropin and spexin. Exp. Biol. Med. [Internet]. 2021; 246(17):1917-1927. doi: https://doi.org/gq8fsz DOI: https://doi.org/10.1177/15353702211012417
Pałasz A, Żarczyński P, Bogus K, Mordecka–Chamera K, Della Vecchia A, Skałbania J, Worthington JJ, Krzystanek M, Żarczyńska M. Modulatory effect of olanzapine on SMIM20/ phoenixin, NPQ/spexin and NUCB2/nesfatin–1 gene expressions in the rat brainstem. Pharmacol. Rep. [Internet]. 2021; 73(4):1188-1194. doi: https://doi.org/ph3f DOI: https://doi.org/10.1007/s43440-021-00267-7
Kalezić N, Stojanović M, Dimić N, Jovanović K, Trailović R, Obrenović KB. Hipertenzivna kriza – dijagnoza i lečenje [Hypertensive crisis: Diagnosis and treatment]. Galenika Med. J. [Internet]. 2022; 1(3):42-47. Serbian. doi: https://doi.org/ph3g DOI: https://doi.org/10.5937/Galmed2203042K
El–Saka MH, Abo El Gheit RE, El Saadany A, Alghazaly GM, Marea KE, Madi NM. Effect of spexin on renal dysfunction in experimentally obese rats: potential mitigating mechanisms via galanin receptor–2. Arch. Physiol. Biochem. [Internet]. 2023; 129(4):933-942. doi: https://doi.org/ph3h DOI: https://doi.org/10.1080/13813455.2021.1887265
Tavakoli A, Aliakbari F, Mehranjani MS. Kisspeptin decreases the adverse effects of human ovarian vitrification by regulating ROS–related apoptotic occurrences. Zygote [Internet]. 2023; 31(6):537-543. doi: https://doi.org/ph3j DOI: https://doi.org/10.1017/S0967199423000412
Lysiak JJ. The role of tumor necrosis factor–alpha and interleukin–1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod. Biol. Endocrinol. [Internet]. 2004; 2:1-10. doi: https://doi.org/df5ph6 DOI: https://doi.org/10.1186/1477-7827-2-9