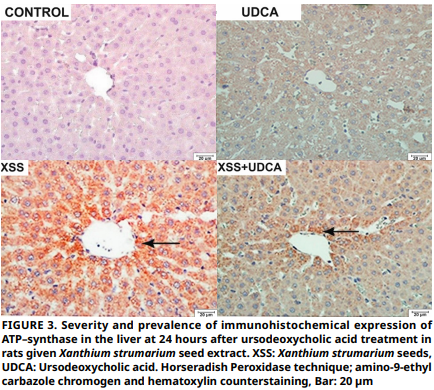
Ursodeoxycholic acid alleviates Xanthium Strumarium induced hepatic and renal toxicity in rats by inhibiting mitochondrial pore opening
Abstract
In Xanthium strumarium toxicity, mitochondrial dysfunction resulting from the opening of mitochondrial pores is identified as the primary mechanism responsible for liver and kidney damage. Ursodeoxycholic acid is known to block mitochondrial pore opening; therefore, this study aims to elucidate the time–dependent therapeutic effect of ursodeoxycholic acid on mitochondrial damage and associated liver and kidney injury in response to X. strumarium exposure. Following the extraction process, Sprague– Dawley rats were administered X. strumarium seed extract (100 g·kg-1) via gavage. Ursodeoxycholic acid was administered via oral gavage 6 hours following the administration of the extract, with continued administration over a 7-day period. In conclusion, the toxic effect of X. strumarium was mitigated by ursodeoxycholic acid, which reduced ATP synthase expression, oxidative damage, mitochondrial Ca2+ concentration, and the opening of mitochondrial pores. Ursodeoxycholic acid mitigated the histopathological toxicity induced by X. strumarium, resulting in a reduction in blood glucose, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, lactate dehydrogenase, blood urea nitrogen, and creatine phosphokinase levels that were closer to control levels. Findings obtained indicate that ursodeoxycholic acid, a blocker of mitochondrial pore opening, can prevent mitochondrial dysfunction and minimize X. strumarium toxicity.
Downloads
References
Kamboj A, Soluja AK. Phytopharmacological review of Xanthium strumarium L. (Cocklebur). Int. J. Green Pharm. [Internet]. 2010; 4(3):129-139. doi: https://doi.org/b7jrzh DOI: https://doi.org/10.4103/0973-8258.69154
Das D, Tangjang S. Bio–stabilization of toxic weeds (Xanthium strumarium and Lantana camara) implementing mono – and polyculture of Eisenia fetida and Eudrilus eugeniae. Environ Sci. Pollut. Res. Int. [Internet]. 2024; 31(37):49891-49904. doi: https://doi.org/pgpk DOI: https://doi.org/10.1007/s11356-024-34487-0
Machado M, Queiroz CRR, Wilson TM, Sousa DER, Castro MB, Saravia A, Lee ST, Armien AG, Barros SS, Riet–Correa F. Endemic Xanthium strumarium poisoning in cattle in flooded areas of the Araguari River, Minas Gerais, Brazil. Toxicon [Internet]. 2021; 200:23-29. doi: https://doi.org/pgpm DOI: https://doi.org/10.1016/j.toxicon.2021.06.019
Sosa S, Capelli A, Corro AC, Dutra F, Santos CGY. Intoxication of dairy cows in Uruguay by ingestion of cocklebur (Xanthium strumarium) seeds in sorghum silage. J. Vet. Diagn. Invest. [Internet]. 2025; 37(1):141-144. doi: https://doi.org/pgpn DOI: https://doi.org/10.1177/10406387241294206
García–Santos C, Capelli A. Plant and mycotoxin poisonings in ruminants diagnosed in Uruguay. Vet. (Montevideo). [Internet]. 2016 [cited 12 Dec. 2024]; 52(202):28-42. Available in: https://goo.su/uGdzF
Saidi H, Mofid M. Toxic Effect of Xanthium strumarium as an Herbal Medicine Preparation. EXCLI J. [Internet]. 2009; 8:115-117. doi: https://doi.org/pgpp
Turgut M, Alhan CC, Gürgöze M, Kurt A, Doğan Y, Tekatli M, Akpolat N, Aygün AD. Carboxyatractyloside poisoning in humans. Ann. Trop. Paediatr. [Internet]. 2005; 25(2):125-134. doi: https://doi.org/fg93nf DOI: https://doi.org/10.1179/146532805X45728
Gurley ES, Rahman M, Hossain MJ, Nahar N, Faiz MA, Islam N, Sultana R, Khatun S, Uddin MZ, Haider MS, Islam MS, Ahmed BN, Rahman MW, Mondal UK, Luby SP. Fatal outbreak from consuming Xanthium strumarium seedlings during time of food scarcity in northeastern Bangladesh. Plos One [Internet]. 2010; 5(3):e9756. doi: https://doi.org/fjc5bj DOI: https://doi.org/10.1371/journal.pone.0009756
Alves–Figueiredo H, Silva–Platas C, Lozano O, Vázquez–Garza E, Guerrero–Beltrán CE, Zarain–Herzberg A, García–Rivas G. A systematic review of post–translational modifications in the mitochondrial permeability transition pore complex associated with cardiac diseases. Biochim. Biophys. Acta Mol. Basis Dis. [Internet]. 2021; 1867(1):165992. doi: https://doi.org/pgpq DOI: https://doi.org/10.1016/j.bbadis.2020.165992
Nikles S, Heuberger H, Hilsdorf E, Schmücker R, Seidenberger R, Bauer R. Influence of Processing on the Content of Toxic Carboxyatractyloside and Artactyloside and the Microbiological Status of Xanthium sibiricum Fruits (Ceng’erzi). Planta Med. [Internet]. 2015; 81(12-13):1213-1220. doi: https://doi.org/g6n8gb DOI: https://doi.org/10.1055/s-0035-1546207
Keskin–Alkaç Z, Korkak FA, Dağoğlu G, Eröksüz Y, Tanyıldızı S. Tamoxifen and sodium thiosulfate reduces hepatic and renal damage induced by Xanthium strumarium L. Through controlling mitochondrial permeability. Med. Weter. [Internet]. 2025; 81(3):119-127. doi: https://doi.org/pgpr DOI: https://doi.org/10.21521/mw.6974
Kapur A, Ayuso JM, Rehman S, Kumari S, Felder M, Stenerson Z, Skala MC, Beebe D, Barroilhet L, Patankar MS. Oxidative phosphorylation inhibitors inhibit proliferation of endometriosis cells. Reproduction [Internet]. 2023; 165(6):617-628. doi: https://doi.org/pgps DOI: https://doi.org/10.1530/REP-22-0265
Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J. Lipid. Res. [Internet]. 2014; 55(8):1553- 1595. doi: https://doi.org/f6k2hg DOI: https://doi.org/10.1194/jlr.R049437
Rajesh KG, Suzuki R, Maeda H, Yamamoto M, Yutong X, Sasaguri S. Hydrophilic bile salt ursodeoxycholic acid protects myocardium against reperfusion injury in a PI3K/ Akt dependent pathway. J. Mol. Cell. Cardiol. [Internet]. 2005; 39(5):766-776. doi: https://doi.org/ffhm82 DOI: https://doi.org/10.1016/j.yjmcc.2005.07.014
Qi H, Shen D, Jiang C, Wang H, Chang M. Ursodeoxycholic acid protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis in MPTP/MPP+–induced Parkinson’s disease. Neurosci Lett. [Internet]. 2021; 741: 135493. doi: https://doi.org/pgpt
Laurens JB, Bekker LC, Steenkamp V, Stewart MJ. Gas chromatographic–mass spectrometric confirmation of atractyloside in a patient poisoned with Callilepis laureola. J. Chromatogr. B. Biomed. Sci. Appl. [Internet]. 2001; 765(2):127-133. doi: https://doi.org/dkfxz8 DOI: https://doi.org/10.1016/S0378-4347(01)00410-8
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. [Internet]. 1979; 95(2):351–358. doi: https://doi.org/bktx4x DOI: https://doi.org/10.1016/0003-2697(79)90738-3
Ellman GL. Tissue sulphydryl groups. Arch Biochem. Biophys. [Internet]. 1959; 82(1):70–77. doi: https://doi.org/bz2vt8 DOI: https://doi.org/10.1016/0003-9861(59)90090-6
Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. [Internet]. 1988; 34(3):497–500. PMID: 3349599. Available in: https://n9.cl/obr0f3 DOI: https://doi.org/10.1093/clinchem/34.3.497
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J. Biol. Chem. [Internet]. 1951; 193(1):265–275. PMID: 14907713. Available in: https://n9.cl/nrvmy DOI: https://doi.org/10.1016/S0021-9258(19)52451-6
Wang LL, Yu QL, Han L, Ma XL, Song RD, Zhao SN, Zhang WH. Study on the effect of reactive oxygen spesies–mediated oxidative stress on the activation of mitochondrial apoptosis and the tenderness of yak meat. Food Chem. [Internet]. 2018; 244:394-402. doi: https://doi.org/pgpv DOI: https://doi.org/10.1016/j.foodchem.2017.10.034
Wang LL, Han L, Ma XL. Yu QL, Zhao SN. Effect of mitochondrial apoptotic activation through the mitochondrial membrane permeability transition pore on yak meat tenderness during postmortem aging. Food Chem. [Internet]. 2017; 234:323-331. doi: https://doi.org/g7fw42 DOI: https://doi.org/10.1016/j.foodchem.2017.04.185
Hu ZG, Zhou L, Ding SZ. Effect of aerobic training to exhaustive exercise rat mitochondrial permeability transition pore. J. Shenyang Sport Univ. [Internet]. 2015; 34(3):64-67. Available in: https://goo.su/JVvutD
Steenkamp PA, Harding NM, Van–Heerden FR, van–Wyk BE. Determination of atractyloside in Callilepis laureola using solid–phase extraction and liquid chromatography– atmospheric pressure ionisation mass spectrometry. J. Chromatogr A. [Internet]. 2004; 1058(1-2):153-162. doi: https://doi.org/ddx7q2 DOI: https://doi.org/10.1016/S0021-9673(04)01305-6
Alkaç ZK, Korkak FA, Dağoğlu G, İncili CA, Hark BD, Tanyıldızı S. Puerarin mitigates oxidative injuries, opening of mitochondrial permeability transition pores and pathological damage associated with liver and kidney in Xanthium strumarium– intoxicated rats. Toxicon [Internet]. 2022; 213:13-22. doi: https://doi.org/pgpx DOI: https://doi.org/10.1016/j.toxicon.2022.04.004
Koprdova R, Osacka J, Mach M, Kiss A. Acute Impact of Selected Pyridoindole Derivatives on Fos Expression in Different Structures of the Rat Brain. Cell Mol Neurobiol. [Internet]. 2018; 38(1):171-180. doi: https://doi.org/gcwvg6 DOI: https://doi.org/10.1007/s10571-017-0520-2
Dou JP, Wu Q, Fu CH, Zhang DY, Yu J, Meng XW, Liang P. Amplified intracellular Ca 2+ for synergistic anti–tumor therapy of microwave ablation and chemotherapy. J. Nanobiotechnology [Internet]. 2019; 17:1-17. doi: https://doi.org/gp5dqc DOI: https://doi.org/10.1186/s12951-019-0549-0
Nolfi–Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. [Internet]. 2020; 37:101674. doi: https://doi.org/gmxqv7 DOI: https://doi.org/10.1016/j.redox.2020.101674
Wang Y, Han T, Xue M, Han P, Zhang QY, Huang BK, Zhang H, Ming QL, Peng W, Qin LP. Hepatotoxicity of kaurene glycosides from Xanthium strumraium L. fruits in mice. Pharmazie. [Internet]. 2011; 66(6):445-449. doi: https://doi.org/pgpz
Liu R, Shi D, Zhang J, Li X, Han X, Yao X, Fang J. Xanthatin Promotes Apoptosis via Inhibiting Thioredoxin Reductase and Eliciting Oxidative Stress. Mol. Pharm. [Internet]. 2018; 15(8):3285-3296. doi: https://doi.org/gdsvwj DOI: https://doi.org/10.1021/acs.molpharmaceut.8b00338
Atlante A, Valenti D, Latina V, Amadoro G. Dysfunction of Mitochondria in Alzheimer’s Disease: ANT and VDAC Interact with Toxic Proteins and Aid to Determine the Fate of Brain Cells. Int. J. Mol. Sci. [Internet]. 2022; 23(14):7722. doi: https://doi.org/pgp3 DOI: https://doi.org/10.3390/ijms23147722
Nirody JA, Budin I, Rangamani P. ATP synthase: Evolution, energetics, and membrane interactions. J. Gen. Physiol. [Internet]. 2020; 152(11):e201912475. doi: https://doi.org/g89s3m DOI: https://doi.org/10.1085/jgp.201912475
Campanella M, Parker N, Tan CH, Hall AM, Duchen MR. IF1: setting the pace of the F1F0-ATP synthase. Trends. Biochem. Sci. [Internet]. 2009; 34(7):343-350. doi: https://doi.org/cpcpn3 DOI: https://doi.org/10.1016/j.tibs.2009.03.006
Grover GJ, Atwal KS, Sleph PG, Wang FL, Monshizadegan H, Monticello T, Green DW. Excessive ATP hydrolysis in ischemic myocardium by mitochondrial F1F0-ATPase; effect of selective pharmacological inhibition of mitochondrial ATPase hydrolase activity. Am. J. Physiol. Heart. Circ. Physiol. [Internet]. 2004; 287(4):H1747-H1755. doi: https://doi.org/d98427 DOI: https://doi.org/10.1152/ajpheart.01019.2003
Koc S, Aktas A, Sahin B, Ozer H, Zararsiz GE. Protective effect of ursodeoxycholic acid and resveratrol against tacrolimus induced hepatotoxicity. Biotech. Histochem. [Internet]. 2023; 98(7):471-478. doi: https://doi.org/pgp4 DOI: https://doi.org/10.1080/10520295.2023.2228697
Simental–Mendía M, Sánchez–García A, Simental–Mendía LE. Effect of ursodeoxycholic acid on liver markers: A systematic review and meta–analysis of randomized placebo–controlled clinical trials. Br. J. Clin. Pharmacol. [Internet]. 2020; 86(8):1476-1488. doi: https://doi.org/pgp5 DOI: https://doi.org/10.1111/bcp.14311
Rajagopala SV, Singh H, Yu Y, Zabokrtsky KB, Torralba MG, Moncera KJ, Pieper R, Sender L, Nelson KE. Persistent gut microbial dysbiosis in children with acute lymphoblastic leukemia (ALL) during chemotherapy. Microb. Ecol. [Internet]. 2020; 79:1034-1043. doi: https://doi.org/gmwp4f DOI: https://doi.org/10.1007/s00248-019-01448-x
Qi H, Shen D, Jiang C, Wang H, Chang M. Ursodeoxycholic acid protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis in MPTP/MPP+–induced Parkinson’s disease. Neurosci. Lett. [Internet]. 2021; 741:135493. doi: https://doi.org/pgpt DOI: https://doi.org/10.1016/j.neulet.2020.135493
Ali FEM, Hassanein EHM, Bakr AG, El–Shoura EAM, El–Gamal DA, Mahmoud AR, Abd–Elhamid TH. Ursodeoxycholic acid abrogates gentamicin–induced hepatotoxicity in rats: Role of NF–KB–p65/TNF–a, Bax/Bcl–xl/Caspase-3, and eNOS/ iNOS pathways. Life Sci. 2020; 254:117760. doi: https://doi.org/gt5kh7 DOI: https://doi.org/10.1016/j.lfs.2020.117760
Xue LM, Zhang QY, Han P, Jiang YP, Yan RD, Wang Y, Rahman K, Jia M, Han T, Qin LP. Hepatotoxic constituents and toxicological mechanism of Xanthium strumarium L. fruits. J. Ethnopharmacol. [Internet]. 2014; 152(2):272-282. doi: https://doi.org/f5wjfd DOI: https://doi.org/10.1016/j.jep.2013.12.024
Deng Z, He M, Hu H, Zhang W, Zhang Y, Ge Y, Ma T, Wu J, Li L, Sun M, An S, Li J, Huang Q, Gong S, Zhang J, Chen Z, Zeng Z. Melatonin attenuates sepsis–induced acute kidney injury by promoting mitophagy through SIRT3-mediated TFAM deacetylation. Autophagy [Internet]. 2024; 20(1):151-165. doi: https://doi.org/gsqgnt DOI: https://doi.org/10.1080/15548627.2023.2252265
Li L, Han W, Gu Y, Qiu S, Lu Q, Jin J, Luo J, Hu X. Honokiol induces a necrotic cell death through the mitochondrial permeability transition pore. Cancer Res. [Internet]. 2007; 67(10):4894-4903. doi: https://doi.org/c7nj9s DOI: https://doi.org/10.1158/0008-5472.CAN-06-3818
Xu H, Sun Y, Zhang Y, Wang W, Dan J, Yao J, Chen H, Tian F, Sun X, Guo S, Tian Z, Tian Y. Protoporphyrin IX induces a necrotic cell death in human THP-1 macrophages through activation of reactive oxygen species/c–Jun N–terminal protein kinase pathway and opening of mitochondrial permeability transition pore. Cell. Physiol. Biochem. [Internet]. 2014; 34(6):1835- 1848. doi: https://doi.org/f6vtq4 DOI: https://doi.org/10.1159/000366383