Isolation and Molecular Characterization of Bovine Viral Diarrhea Virus in Calves with Congenital Malformation in Türkiye
Abstract
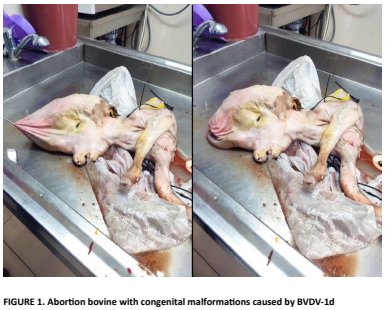
This study aims to identify the causative agent in cases of abortion on a cattle farm in Erzurum province, Türkiye. Samples from the farm were sent to the Erzurum Veterinary Control Institute, Ministry of Agriculture and Forestry. The analysis revealed the presence of Bovine Viral Diarrhea Virus (BVDV) in a newborn calf exhibiting congenital malformations. Subsequently, blood and serum samples were collected for four weeks post-abortion to assess the acute/persistent infection status on the farm. Using the ELISA method, antigen, and antibody positivity were detected in both the cattle and the aborted calf. Six blind passages were conducted in the MDBK cell line to isolate the virus from cerebrospinal fluid. Confirmation of isolation was carried out through regular CT increase in Real-Time RT-PCR due to the non-cytopathogenic nature of the detected virus. The isolate (EVE-BVDV-2023) was identified as belonging to the BVDV-1d genotype through partial genome analysis of the 5’UTR gene. This study conclusively confirms the presence of BVDV infection in cattle herds in the eastern region of Türkiye, particularly in Erzurum province. Future studies should continue efforts to control and eradicate infectious agents in cattle herds, with a particular emphasis on addressing BVDV infection.
Downloads
References
Vilcek S, Nettleton PF. Pestiviruses in wild animals. Vet. Microbiol. [Internet]. 2006; 116(1–3):1–12. doi: https://doi.org/fhh5b8 DOI: https://doi.org/10.1016/j.vetmic.2006.06.003
Yesilbag K, Forster C, Bank-Wolf B, Yılmaz Z, Alkan F, Ozkul A, Burgu I, Rosales, SC, Thiel HJ, Konig M. Genetic heterogeneity of bovine viral diarrhoea virus (BVDV) Isolates from Turkey: Identification of a New Subgroup in BVDV-1. Vet. Microbiol. [Internet]. 2008; 130(3–4):258–267. doi: https://doi.org/bnzgs9 DOI: https://doi.org/10.1016/j.vetmic.2008.01.016
Wu Y, Zhang G, Jiang H, Xin T, Jia L, Zhang Y, Yang Y, Qin T, Xu C, Cao J, Ameni G, Ahmad A, Ding J, Li L, Ma Y, Fan X. Molecular Characteristics of Bovine Viral Diarrhea Virus Strains Isolated from Persistently Infected Cattle. Vet .Sci. [Internet]. 2023; 10(7):413. https://doi.org/n44x DOI: https://doi.org/10.3390/vetsci10070413
Sevik M. The Role of Pestiviruses (BDV and BVDV) in Ruminant Abortion Cases in the Afyonkarahisar Province. Kocatepe Vet. J. [Internet]. 2018; 11(3):238-244. doi: https://doi.org/n4qd
Spetter MJ, Louge-Uriarte EL, Armendano JI, Morrell EL, Canton GJ, Verna AE, Dorsch MA, Pereyra SB, Odeón AC, Saliki JT, González-Altamiranda EA. Detection methods and characterization of bovine viral diarrhea virus in aborted fetuses and neonatal calves over a 22- year period. Brazilian J. Microbiol. [Internet]. 2020; 51(4):2077–2086. doi: https://doi.org/n4qg DOI: https://doi.org/10.1007/s42770-020-00296-z
Britto-de Oliveira PS, Silva-Júnior JVJ, Weiblen R, Flores EF. A new (old) bovine viral diarrhea virus 2 subtype: BVDV-2e. Arch. Virol. [Internet]. 2022; 167(12):2545–2553. doi: https://doi.org/n4qk DOI: https://doi.org/10.1007/s00705-022-05565-w
Sarikaya B, Azkur AK, Gazyagci S, Aslan ME. Genetic variability of bovine viral diarrhea virus in the 5’-UTR in the Central Anatolia of Turkey. Acta. Sci. Vet. [Internet]. 2012[cited 22 Sept 2024]; 40(1):1013. Available in: https://n9.cl/nbedfm
Gürçay M, Kececi H, Öztürk M. Determination of presence and prevalence of bovine viral diarrhea virus ınfection in cattle herds in Bingöl province. Etlik. Vet. Mikrobiyol. Derg. [Internet]. 2020; 31(1):34–38. doi: https://doi.org/n4q2 DOI: https://doi.org/10.35864/evmd.658433
Postel A, Smith DB, Becher P. Proposed update to the taxonomy of pestiviruses: eight additional species within the genus pestivirus, family flaviviridae. Viruses. [Internet]. 2021; 13(8):1-12. doi: https://doi.org/n4q3 DOI: https://doi.org/10.3390/v13081542
Timurkan MO, Aydın H. Increased genetic diversity of BVDV strains circulating in Eastern Anatolia, Türkiye: first detection of BVDV-3 in Turkey. Trop. Anim. Health. Prod. [Internet]. 2019; 7(51):1953–1961. doi: https://doi.org/n4q4 DOI: https://doi.org/10.1007/s11250-019-01901-6
Weber MN, Silveira S, Machado G, Groff FHS, Mosena ACS, Budaszewski RF, Dupont PM, Corbellini LG, Canal CW. High frequency of bovine viral diarrhea virus type 2 in Southern Brazil. Virus. Res. [Internet]. 2014; 191:117–124. doi: https://doi.org/f6k9ng DOI: https://doi.org/10.1016/j.virusres.2014.07.035
Brodersen BW. Bovine Viral Diarrhea Virus Infections: Manifestations of Infection and Recent Advances in Understanding Pathogenesis and Control. Vet. Pathol. [Internet]. 2014; 51(2):453–464. doi: https://doi.org/ghdcvv DOI: https://doi.org/10.1177/0300985813520250
Dogan F, Dagalp SB. Sığırlarda viral nedenli abort olgularının etiyopatogenezi. Sağlık Bilim Enst. Derg. [Internet]. 2017; 5(1):66–77. doi: https://doi.org/n4q5 DOI: https://doi.org/10.24998/maeusabed.310964
Garoussi MT, Mehrzad J, Nejati A. Investigation of persistent infection of bovine viral diarrhea virus (BVDV) in Holstein dairy cows. Trop. Anim. Health. Prod. [Internet]. 2019; 51(4):853–858. doi: https://doi.org/n4q6 DOI: https://doi.org/10.1007/s11250-018-1765-6
Tuncer-Göktuna P, Yesılbag K. Bovine viral diarrhea virus propagates in cell culture after ınoculation of ınactivated sera from persistently ınfected calves. Uludag. Univ. Vet. Fak. Derg. [Internet]. 2016; 35(1-2):7–10. doi: https://doi.org/g7gmps
Cagırgan AA, Kaplan M, Pekmez K, Van-Schalkwyk A, Arslan F, Timurkan MO. The Status of Bovine Viral Diarrhea Virus (BVDV) in Western Türkiye: Detection of Three Subtypes. Kafkas. Univ. Vet. Fak. Derg. [Internet]. 2022; 28(6):709-715. doi: https://doi.org/n4q9
Atasever A, Mendil AS, Timurkan MO. Detection of bovine viral diarrhea virus and bovine herpes virus type 1 in cattle with and without endometritis. Vet. Res. Forum. [Internet]. 2023; 14(10):541-548. doi: https://doi.org/n4rb
İssi M, Gülaçtı İ, Kızıl Ö, Karapınar T, Bulut H, Gül Y. The mucosal disease cases observed in our clinic and confirmed with reverse transcription -polymerase chain reaction (RT-PCR). Fırat. Univ. Sağlık. Bilim. Vet. Derg. [Internet]. 2006[Cited 22 Sept 2024]; 20(3):253-258. Available in: https://goo.su/noqHr
Usta Z, Dıstl O. Hydrocephalus internus and other neural malformations, brachyury in German Holstein twin calves. MAEU. Vet. Fak. Derg. [Internet]. 2019; 4(1):37–40. doi: https://doi.org/n4rf DOI: https://doi.org/10.24880/maeuvfd.492354
Ozkaraca M, Timurkan MO. Erzurum Yöresinde Pnömonili Sığırlarda BVDV’ nin Teşhisinde İmmunohistokimya ve PCR Yöntemleri. Van Vet. J. [Internet]. 2016[Cited 02 July 2024]; 27(2):85-89. Available in: https://goo.su/Nd4vl
Baxi M, McRae D, Baxi S, Greiser-Wilke I, Vilcek S, Amoako K, Deregt D. A one-step multiplex real-time RT-PCR for detection and typing of bovine viral diarrhea viruses. Veterinary Microbiology [Internet]. 2006; 116(1-3), 37-44. https://doi.org/10.1016/j.vetmic.2006.03.026 DOI: https://doi.org/10.1016/j.vetmic.2006.03.026
Ozkul A. The role on infertility eases of IBR/IPV and BVD virus infections eneountered in dairy eattle herds. Ankara Univ. Vet. Fak. Derg. [Internet]. 1995; 42(03):381-387. doi: https://doi.org/n4rj
Vilček S, Herring AJ, Herring JA, Nettleton PF, Lowings JP, Paton DJ. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch. Virol. [Internet]. 1994; 136:309-323. doi: https://doi.org/cmvdqh DOI: https://doi.org/10.1007/BF01321060
Tamura K, Stecher G, Kumar S. MEGA11: Molecular evolutionary genetics analysis, Version 11. Mol. Biol. Evol. [Internet]. 2021; 38(7):3022–3027. doi: https://doi.org/gkknc5 DOI: https://doi.org/10.1093/molbev/msab120
Bianchi MV, Konradt G, de Souza SO, Bassuino DM, Silveira S, Mosena ACS, Canal CW, Pavarini SP, Driemeier D. Natural outbreak of BVDV-1d–ınduced mucosal disease lacking ıntestinal lesions. Vet. Pathol. [Internet]. 2017; 54(2):242–248. doi: https://doi.org/n4rk DOI: https://doi.org/10.1177/0300985816666610
Walz PH, Grooms DL, Passler T, Ridpath JF, Tremblay R, Step DL, Callan RJ, Givens MD. Control of bovine viral diarrhea virus in ruminants. J. Vet. Intern. Med. [Internet]. 2010; 24(3):476–486. doi: https://doi.org/ctv3p5 DOI: https://doi.org/10.1111/j.1939-1676.2010.0502.x
Erol N, Gur S, Acar A. A Serological ınvestigation for bovine viral diarrhea virus ınfection in and around Afyonkarahisar province, West Anatolia. Kocatepe. Vet. J. [Internet]. 2014; 7(2):17–21. doi: https://doi.org/n4rm DOI: https://doi.org/10.5578/kvj.8309
Deng M, Chen N, Guidarini C, Xu Z, Zhang J, Cai L, Yuan S, Sun Y, Metcalfe L. Prevalence and genetic diversity of bovine viral diarrhea virus in dairy herds of China. Vet. Microbiol. [Internet]. 2020; 242:108565. doi: https://doi.org/gtjqvt DOI: https://doi.org/10.1016/j.vetmic.2019.108565
Yesilbag K, Alpay G, Tuncer P. Control and Elimination of Bovine Viral Diarrhoea Virus in a Dairy Herd. Uludag. Univ. Vet. Fak. Derg. [Internet]. 2012[cited 24 Sept 2024]; 31(1):11–17. Available in: https://goo.su/vVycKd
Yesilbag K, Alpay G, Becher P. Variability and global distribution of subgenotypes of bovine viral diarrhea virus. Viruses. [Internet]. 2017; 9(128):1-19. doi: https://doi.org/gsk824 DOI: https://doi.org/10.3390/v9060128
Uttenthal A, Stadejek T, Nylın B. Genetic diversity of bovine viral diarrhoea viruses (BVDV) in denmark during a 10-year eradication period. Acta. Path. Microbiol. Immunol. Scand. [Internet]. 2005; 113(7–8):536–541. doi: https://doi.org/fbr56n DOI: https://doi.org/10.1111/j.1600-0463.2005.apm_227.x
Kuta A, Polak MP, Larska M, Żmudziński JF. Predominance of bovine viral diarrhea virus 1b and 1d subtypes during eight years of survey in Poland. Vet. Microbiol. [Internet]. 2013; 166(3–4):639–644. doi: https://doi.org/f5cfkd DOI: https://doi.org/10.1016/j.vetmic.2013.07.002
Mirosław P, Polak M. Increased Genetic Variation of Bovine Viral Diarrhea Virus in Dairy Cattle in Poland. BMC. Vet. Res. [Internet]. 2019; 15(1):1–12. doi: https://doi.org/g6cdgc DOI: https://doi.org/10.1186/s12917-019-2029-z
Kucer N, Markovıc E, Prpıc J, Rudan D, Jemersıc L, Hajek Z, Skaro K, Pavlak M, Rudan N. The Epidemiology of bovine viral diarrhea virus ınfection on a dairy farm-clinical signs, seroprevalence, virus detection and genotyping. Vet. Arch. [Internet]. 2022; 92(2):119–126. doi: https://doi.org/n4rn DOI: https://doi.org/10.24099/vet.arhiv.1028
Scharnböck B, Roch FF, Richter V, Funke C, Firth CL, Obritzhauser W, Baumgartner W, Käsbohrer A, Pinior B. A meta-analysis of bovine viral diarrhoea virus (BVDV) prevalences in the global cattle population. Sci. Rep. [Internet]. 2018; 8(14420):1-15. doi: https://doi.org/gfchz9 DOI: https://doi.org/10.1038/s41598-018-32831-2
Fulton RW, Ridpath JF, Saliki JT, Briggs RE, Confer AW, Burge LJ, Purdy CW, Loan RW, Duff GC, Payton ME. Bovine viral diarrhea virus (BVDV) 1b: predominant bvdv subtype in calves with respiratory disease. Can. J. Vet. Res. [Internet]. 2002; 66(3):181-190. PMID: 12146890. Available in: https://pubmed.ncbi.nlm.nih.gov/12146890/
Vuuren MV. Bovine viral diarrhoea virus ınfection in livestock in southern Africa. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. [Internet]. 2006; 1(9):1-5. doi: https://doi.org/fnhkbp DOI: https://doi.org/10.1079/PAVSNNR20061009
Nogueira FCS, Velasquez E, Melo ASO, Domont GB. Zika virus may not be alone: proteomics associates a bovine-like viral diarrhea virus to microcephaly. Bio. Rxiv. [Internet]. 2015; 21:1885-1886. doi: https://doi.org/bm4c DOI: https://doi.org/10.1101/062596
Alpay G, Toker EB, Yeşilbağ K. Persistent BVD virus ınfections in offspring from imported heifers. Trop Anim. Health. Prod. [Internet]. 2019; 51(2):297–302. doi: https://doi.org/n4rp DOI: https://doi.org/10.1007/s11250-018-1685-5
Oğuzoğlu TC, Muz D, Yılmaz V, Timurkan MO, Alkan F, Akça Y, Burgu I. Molecular characteristics of bovine virus diarrhoea virus 1 ısolates from Turkey: approaches for an eradication programme. Transbound. Emerg. Dis. [Internet]. 2012; 59(4):303–310. doi: https://doi.org/d56k57 DOI: https://doi.org/10.1111/j.1865-1682.2011.01272.x
Toker EB, Yeşilbağ K. Molecular characterization and comparison of diagnostic methods for bovine respiratory viruses (BPIV-3, BRSV, BVDV, and BoHV-1) in field samples in Northwestern Turkey. Trop. Anim. Health. Prod. [Internet]. 2021; 53(79):1-11. doi: https://doi.org/n4rq DOI: https://doi.org/10.1007/s11250-020-02489-y
Klemens O, Dubrau D, Tautz N. Characterization of the determinants of NS2-3-ındependent virion morphogenesis of pestiviruses. J. Virol. [Internet]. 2015; 89(22):11668–11680. doi: https://doi.org/g8dsjh DOI: https://doi.org/10.1128/JVI.01646-15