Efecto de una vacuna clostridial combinada sobre los niveles de citocinas e inmunoglobulinas en corderos
Resumen
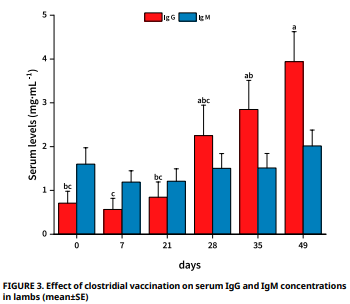
Las infecciones causadas por agentes clostridiales son difíciles de tratar y tienen una alta tasa de mortalidad. Este estudio tuvo como objetivo determinar los efectos de la dosis recomendada de una vacuna clostridial comercial en los niveles séricos del TNF–α, IL–1β, IL–6 e IL–10, e inmunoglobulinas IgG e IgM en corderos. Una sola vacuna clostridial comercial, que contenía Clostridium perfringens tipos A, B, C y D, Clostridium novyi tipo B, Clostridium septicum, Clostridium tetani y Clostridium chauvoei, se administró por vía subcutánea (2 mL por dosis) a 15 corderas (de 2 a 3 meses de edad), con dos dosis administradas en un intervalo de cuatro semanas. Se tomaron muestras de sangre a las 0, 2, 4, 8, 12 y 24 horas después de la primera dosis, y se midieron los niveles séricos específicos de especie de TNF–α, IL–1β, IL–6 e IL–10 utilizando kits ELISA comerciales. Los niveles séricos de IgG e IgM se determinaron utilizando un lector ELISA a partir de muestras de sangre tomadas los días 0, 7 y 21 después de cada una de la primera y segunda vacunación. Si bien no se observaron cambios estadísticos en los niveles de IL–1β e IL–10 (P>0,05), el TNF–α alcanzó su punto máximo en la segunda hora (P<0,05) y los niveles de IL–6 alcanzaron los niveles más altos en la octava y duodécima hora después de la vacunación (P<0,05). Aun cuando no se detectaron cambios en los niveles de IgM en el estudio (P>0,05), los niveles de IgG se elevaron significativamente a los 7 y 21 días posteriores a la revacunación (P<0,05). En conclusión, la aplicación de la vacuna clostridial en corderos estimula la síntesis de varias citoquinas y eleva la respuesta IgG responsable de la inmunidad después de la segunda aplicación.
Descargas
Citas
Lewis CJ. Control of important clostridial diseases of sheep. Vet. Clin. North Am. – Food Anim. Pract. [Internet]. 2011; 27:121–126. doi: https://doi.org/c4zm7m DOI: https://doi.org/10.1016/j.cvfa.2010.10.009
Hadimli H. Bacterial diseases in calves. In: Erdem H, editor. Calf health and husbandry in preventing calf losses. [Internet]. Ankara (Türkiye): Medisan; 2021 [cited May 15, 2025]. p. 73–79. Available in: https://goo.su/EbeqC
Simpson KM, Callan RJ, Van Metre DC. Clostridial abomasitis and enteritis in ruminants. Vet. Clin. North Am. – Food Anim. Pract. [Internet]. 2018; 34:155–184. doi: https://doi.org/gc7djv DOI: https://doi.org/10.1016/j.cvfa.2017.10.010
Junior CAO, Silva ROS, Lobato FCF, Navarro MA, Uzal FA. Gas gangrene in mammals: A review. J. Vet. Diagn. Investig. [Internet]. 2020; 32(2):175–183. doi: https://doi.org/gnxckq DOI: https://doi.org/10.1177/1040638720905830
Khiav LA, Zahmatkesh A. Major pathogenic Clostridia in human and progress toward the clostridial vaccines. Iran. J. Basic. Med. Sci. [Internet]. 2022; 25(9):1059–1068. doi: https://doi.org/p8sc
Walker PD. Bacterial vaccines: old and new, veterinary and medical. Vaccine [Internet]. 1992; 10(14):977–990. doi: https://doi.org/bjdtwk DOI: https://doi.org/10.1016/0264-410X(92)90106-T
Sevinc M, Ider M. Infectious necrotic hepatitis (black disease) in ruminants. In: OK M, editor. Clostridial infections of animals. Ankara (Türkiye): Turkiye Klinikleri; 2019. p. 15–19.
Khiav LA, Zahmatkesh A. Vaccination against pathogenic clostridia in animals: A review. Trop. Anim. Health Prod. [Internet]. 2021; 53:284. doi: https://doi.org/p8sd DOI: https://doi.org/10.1007/s11250-021-02728-w
HBS–tarbil. Ruhsatlı Veteriner Biyolojik Ürünler [Licensed Veterinary Biological Products] [Internet]. T.C. Tarim Ve Orman Bakanliği [Republic of Türkiye, Ministry of Agriculture and Forestry]. HBSPORTAL01. 2024 [cited Dec 05, 2024]. Available from: https://goo.su/HRIo5x8
Yazar E. Veterinary drug and vaccine from A to Z. Istanbul (Türkiye): Nobel Tip Press; 2018.
Akdoğan M, Yöntem M. Sitokinler [Cytokines]. Online Türk Sağlık Bilim. Derg. [Internet]. 2018; 3(1):36–45. Turkish. doi: https://doi.org/p8sf DOI: https://doi.org/10.26453/otjhs.350321
Dik B, Dik I, Bahcivan E, Avci O. Corynebacterium cutis lysate treatment can increase the efficacies of PPR vaccine. J. Interferon Cytokine Res. [Internet]. 2016; 36(10):599–606. doi: https://doi.org/f87wk9 DOI: https://doi.org/10.1089/jir.2016.0035
Er A, Corum O, Dik B, Bahcivan E, Eser H, Yazar E. Determination of the effect of Corynebacterium cutis lysate treatment on the cytokine levels in sheep. Eurasian J. Vet. Sci. [Internet]. 2015; 31(4):209–213. doi: https://doi.org/p8sg DOI: https://doi.org/10.15312/EurasianJVetSci.2015413524
Haznedar R, Sözcükler A. Plazma hücreleri ve immünoglobulinler [Plasma cells and immunoglobulins]. Türk Hematoloji Derneği HematoLog. [Internet]. 2013 [cited May 15, 2025]; 3(1):8–19. Turkish. Available in: https://goo.su/zq4Ir
Yilmaz N, Akgül Y. İmmünglobunlinler ve septisemi [Immunglobulins and Septicemia] Uludağ Üniv. Vet. Fak. Derg. [Internet]. 2014 [cited May 15, 2025]; 33(1,2):33–42. Turkish. Available in: https://goo.su/NEIP
Xu B, Wang J, Zhang M, Wang P, Wei Z, Sun Y, Tao Q, Ren L, Hu X, Guo Y, Fei J, Zhang L, Li N, Zhao Y. Expressional analysis of immunoglobulin D in cattle (Bos taurus), a large domesticated ungulate. PLOS One [Internet]. 2012; 7(9):1–11. doi: https://doi.org/p8sh DOI: https://doi.org/10.1371/journal.pone.0044719
Farshi E. Cytokine storm response to COVID–19 vaccinations. J. Cytokine Biol. [Internet]. 2020 [cited May 21, 2025]; 5(2):34. Available in: https://goo.su/QqM7iK4
Cohen D, Ashkenazi S, Green M, Lerman Y, Slepon R, Robin G, Orr N, Taylor D N, Sadoff JC, Chu C, Shiloach J, Schneerson R, Robbins JB. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect. Immun. [Internet]. 1996; 64(10):4074–4077. doi: https://doi.org/p8sm DOI: https://doi.org/10.1128/iai.64.10.4074-4077.1996
Vervelde L, Bakker N, Kooyman FNJ, Cornelissen AWCA, Bank CMC, Nyame AK, Cummings RD, van Die I. Vaccination– induced protection of lambs against the parasitic nematode Haemonchus contortus correlates with high IgG antibody responses to the LDNF glycan antigen. Glycobiology [Internet]. 2003; 13(11):795–804. doi: https://doi.org/d9pzf3 DOI: https://doi.org/10.1093/glycob/cwg107
Machín C, Corripio–Miyar Y, Hernández JN, Pérez–Hernández T, Hayward AD, Wright HW, Price DRG, Matthews JB, McNeilly TN, Nisbet AJ, González JF. Cellular and humoral immune responses associated with protection in sheep vaccinated against Teladorsagia circumcincta. Vet. Res. [Internet]. 2021; 52(1):89. doi: https://doi.org/p8sn DOI: https://doi.org/10.1186/s13567-021-00960-8
Yazar E. Antibacterial treatment. In: Elmas M, editor. Sheep– Goat Handbook Konya (Türkiye): Billur press; 2013. p. 293–304.
Yazar E. Chemotherapeutics. In: Yazar E, editor. Veterinary drug guide treatment handbook. Ankara (Türkiye): Nobel Tip press; 2024. p. 83–172.
Pasandideh R, Nassiri MR, Raouf–Delgosha M, Aslaminejad AA, Tahmoorespur M, Zibaei S, Doosti M, Pasandideh M. Evaluation of cytokine mRNA expression in vaccinated guinea pigs with foot–and–mouth disease type O inactivated vaccine. Arch. Razi Ins. [Internet]. 2016; 71:15–19. doi: https://doi.org/p8sq
Solfaine R, Fikri F, Maslamama ST, Purnama MTE, Hamid IS. Exploring the protective efficacy of lactobacillus acidophilus against interleukin–1beta (IL–1β) expression in mice induced with canine coronavirus vaccine. Adv. Anim. Vet. Sci. [Internet]. 2024; 12(4):673–678. doi: https://doi.org/p8sr DOI: https://doi.org/10.17582/journal.aavs/2024/12.4.673.678
Yu H, Chen K, Sun Y, Carter M, Garey KW, Savidge TC, Devaraj S, Tessier ME, von Rosenvinge EC, Kelly CP. Cytokines are markers of the Clostridium difficile–induced inflammatory response and predict disease severity. Clin. Vaccine. Immunol. [Internet]. 2017; 24(8):e00037–00017. doi: https://doi.org/gbs6vx DOI: https://doi.org/10.1128/CVI.00037-17
Lalor MK, Floyd S, Gorak–Stolinska P, Ben–Smith A, Weir RE, Smith SG, Newport MJ, Blitz R, Mvula H, Branson K, McGrath N, Crampin AC, Fine PE, Dockrell HM. BCG vaccination induces different cytokine profiles following infant BCG vaccination in the UK and Malawi. J. Infect. Dis. [Internet]. 2011; 204(7):1075–1085. doi: https://doi.org/fq5hpn DOI: https://doi.org/10.1093/infdis/jir515
Asadi A, Khiav LA, Emadi A, Dadar M. Evaluation of humoral immune responses against C. perfringens epsilon toxin in Iranian sheep and goats after vaccination. Vet. Anim. Sci. [Internet]. 2023; 21:100305. doi: https://doi.org/p8ss DOI: https://doi.org/10.1016/j.vas.2023.100305
Rashid BM, Yüksek N. The effects of immunostimulants (zinc, levamisole, vitamin AD3E) use together with enterotoxemia vaccine on immunoglobulins in sheep. Turk. J. Vet. Anim. Sci. [Internet]. 2019 [cited May 18, 2025]; 3(2):57–65. Available in: https://goo.su/16jB4
Tariq M, Anjum AA, Sheikh AA, Awan AR, Ali MA, Sattar MMK, Hussain S, Tehreem A. Preparation and evaluation of alum precipitate and oil adjuvant multivalent vaccines against Clostridium perfringens. Kafkas Univ. Vet. Fak. Derg. [Internet]. 2021; 27(4):475–482. doi: https://doi.org/p8sv
Moosawi M. Production and standardization two types of clostridial–vaccines for sheep and cattle. Arch. Razi Inst. [Internet]. 1988; 38:83–88. doi: https://doi.org/p8sw
Aboudola S, Kotloff KL, Kyne L, Warny M, Kelly EC, Sougioultzis S, Giannasca PJ, Monath TP, Kelly CP. Clostridium difficile vaccine and serum immunoglobulin G antibody response to toxin A. Infect. Immun. [Internet]. 2003; 71(3):1608–1610. doi: https://doi.org/d8gpkb DOI: https://doi.org/10.1128/IAI.71.3.1608-1610.2003
Kotloff KL, Wasserman SS, Losonsky GA, Thomas Jr W, Nichols R, Edelman R, Bridwell M, Monath TP. Safety and immunogenicity of increasing doses of a Clostridium difficile toxoid vaccine administered to healthy adults. Infect. Immun. [Internet]. 2001; 69(2):988–995. doi: https://doi.org/fn7kwn DOI: https://doi.org/10.1128/IAI.69.2.988-995.2001
Scrimin F, Campisciano G, Comar M, Ragazzon C, Davanzo R, Quadrifoglio M, Giangreco M, Stabile G, Ricci G. IgG and IgA antibodies post SARS–CoV–2 vaccine in the breast milk and sera of breastfeeding women. Vaccines. [Internet]. 2022; 10:125. doi: https://doi.org/gn7548 DOI: https://doi.org/10.3390/vaccines10010125
Örmeci AR, Eren E, Kaya S, Kisioglu AN. Changes in immunity level after second and third doses of measles immunization. J. Child Health Dis. [Internet]. 2008 [cited May 05, 2025]; 51(4):199–205. Available in: https://goo.su/wGSKVb