Efecto de la raíz de maca (Lepidium meyenii) sobre algunos parámetros bioquímicos y antioxidantes en ratas con síndrome de ovario poliquístico experimental
Resumen
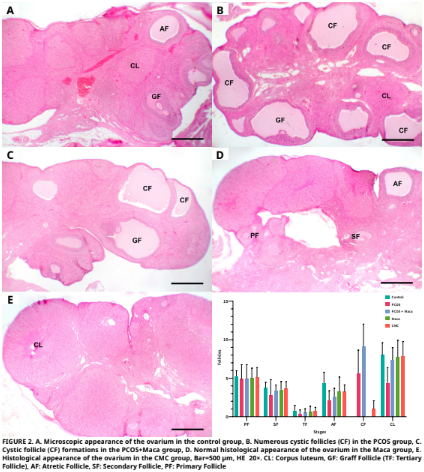
En este estudio se evaluó el posible efecto terapéutico de la raíz de maca (Lepidium meyenii) sobre el daño oxidativo y los cambios histopatológicos en los ovarios inducidos por el síndrome de ovario poliquístico experimental (SOP) en ratas. El estudio involucró a treinta y cinco ratas Sprague Dawley hembras, cada una de 2,5 meses de edad. Estas ratas se dividieron en cinco grupos distintos. El primer grupo no recibió ningún tratamiento ni intervención. Se aplicó carboximetilcelulosa (CMC) (1 %) al segundo grupo. Se aplicó raíz de maca al tercer grupo en una dosis de 2 g·kg-1·día-1 de peso corporal por sonda durante 7 días (d). Se aplicó 1 mg·kg-1·d-1 de letrozol (agente para SOP) disuelto en 1% de CMC al cuarto grupo por sonda durante 21 d. Se aplicaron letrozol y raíz de maca juntos al quinto grupo. La aplicación de raíz de maca se inició el día 15 de la aplicación de letrozol y se aplicó durante 7 d, mientras que el letrozol se aplicó durante 21 d comenzando 14 d antes de la aplicación de raíz de maca y ambas aplicaciones finalizaron el d 22. En el grupo con SOP, se observó que los niveles de malondialdehído (MDA) estaban elevados en comparación con el grupo de control, mientras que los niveles de glutatión reducido (GSH), junto con las actividades de catalasa (CAT), glutatión peroxidasa (GSH–Px), glutatión–S– transferasa (GST) y superóxido dismutasa (SOD), estaban reducidos. En el grupo de SOP + raíz de maca, se determinaron diferencias en comparación con el grupo de SOP aplicado, y se encontró que todos los valores de los parámetros estaban cerca de los valores del grupo de control (excepto GSH–Px y Hormona folículo estimulante (FSH)). El número de folículos atrésicos disminuyó significativamente en el grupo de SOP y el grupo de SOP + maca en comparación con el grupo de control (P≤0.01). Se observó que el número de folículos quísticos aumentó estadísticamente de manera significativa en los grupos de SOP en comparación con los otros grupos (P≤0.001). En el desarrollo de la toxicidad ovárica relacionada con el SOP y el estrés oxidativo, el SOP puede contribuir a una discrepancia entre oxidantes y antioxidantes, mientras que la raíz de maca puede ayudar a aliviar los efectos secundarios graves causados por el SOP.
Descargas
Citas
Yasmin A, Roychoudhury S, Choudhury AP, Ahmed AF, Dutta S, Mottola F, Verma V, Kalita JC, Kumar D, Sengupto P, Kolesarova A. Polycystic ovary syndrome: An updated overview foregrounding impacts of ethnicities and geographic variations. Life [Internet]. 2022; 12(12):1974. doi: https://doi.org/pfx4 DOI: https://doi.org/10.3390/life12121974
Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J. Clin. Endocrinol. Met. [Internet]. 2021; 106(3):e1071–e1083. doi: https://doi.org/gqnwgb DOI: https://doi.org/10.1210/clinem/dgaa839
The Rotterdam ESHRE/ASRM–Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long–term health risks related to polycystic ovary syndrome. Fertil. Steril. [Internet]. 2003; 81(1):19–25. doi: https://doi.org/cd5736 DOI: https://doi.org/10.1016/j.fertnstert.2003.10.004
Rudnicka E, Suchta K, Grymowicz M, Calik–Ksepka A, Smolarczyk K, Duszewska AM, Smolarczyk R, Meczekalski, B. Chronic low grade inflammation in pathogenesis of PCOS. Int. J. Mol. Sci. [Internet]. 2021; 22(7):3789. doi: https://doi.org/gpvzt3 DOI: https://doi.org/10.3390/ijms22073789
Xu Y, Qiao J. Association of insulin resistance and elevated androgen levels with polycystic ovarian syndrome (PCOS): a review of literature. J. Healthc. Eng. [Internet]. 2022; 2022(1):9240569. doi: https://doi.org/grqdz2 DOI: https://doi.org/10.1155/2022/9240569
Pruteanu LL, Bailey DS, Grădinaru AC, Jäntschi L. The biochemistry and effectiveness of antioxidants in food, fruits, and marine algae. Antioxidants [Internet]. 2023; 12(4):860. doi: https://doi.org/pfx7 DOI: https://doi.org/10.3390/antiox12040860
Averill–Bates D. Reactive oxygen species and cell signaling. Review. BBA Mol. Cell Res. [Internet]. 2024; 1871(2):119573. doi: https://doi.org/pfx8 DOI: https://doi.org/10.1016/j.bbamcr.2023.119573
Wattanapitayakul SK, Bauer JA. Oxidative pathways in cardiovascular disease: roles, mechanisms, and therapeutic implications. Pharmacol. Ther. [Internet]. 2001; 89(2):187–206. doi: https://doi.org/dpd886 DOI: https://doi.org/10.1016/S0163-7258(00)00114-5
Liang J, Gao Y, Feng Z, Zhang B, Na Z, Li D. Reactive oxygen species and ovarian diseases: Antioxidant strategies. Redox Biol. [Internet]. 2023; 62:102659. doi: https://doi.org/gskdhw DOI: https://doi.org/10.1016/j.redox.2023.102659
Gonzales GF, Nieto J, Rubio J, Gasco M. Effect of Black maca (Lepidium meyenii) on one spermatogenic cycle in rats. Andrologia [Internet]. 2006; 38(5):166–172. doi: https://doi.org/dsq5c9 DOI: https://doi.org/10.1111/j.1439-0272.2006.00733.x
Yildirim G, Rukset A, Ozkan F, Kumbak B, Ficicioglu C, Yesildaglar N. The effects of letrozole and melatonin on surgically induced endometriosis in a rat model: A preliminary study. Fertil. Steril. [Internet]. 2010; 93(6):1787–1792. doi: https://doi.org/dsndjk DOI: https://doi.org/10.1016/j.fertnstert.2009.09.021
Placer ZA, Cushman L, Johnson BC. Estimation of products of lipid peroxidation in biological fluids. Anal. Biochem. [Internet]. 1966; 16(2):359–364. doi: https://doi.org/b96rpj DOI: https://doi.org/10.1016/0003-2697(66)90167-9
Ellman GL, Courtney KD, Andres Jr V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. [Internet]. 1961; 7(2):88–95. doi: https://doi.org/fwdkkz DOI: https://doi.org/10.1016/0006-2952(61)90145-9
Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 2nd. ed. Weinheim (Germany): Verlag Chemie; 1974. p. 673–678. DOI: https://doi.org/10.1016/B978-0-12-091302-2.50032-3
Beutler E. Red Cell Metabolism. A Manual of Biochemical Methods, 3rd. ed. Orlando (USA): Grune & Stratton; 1984. p. 310–311.
Habig WH, Pabst MJ, Jakoby WB. Glutathione S–transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. [Internet]. 1974; 249(22):7130–7139. doi: https://doi.org/gjjzqq DOI: https://doi.org/10.1016/S0021-9258(19)42083-8
Sun YI, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. [Internet]. 1988; 34(3): 497–500. PMID: 3349599. Available in: https://n9.cl/obr0f3 DOI: https://doi.org/10.1093/clinchem/34.3.497
Lowry O, Rosebrough N, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. [Internet]. 1951; 193(1):265–275. doi: https://doi.org/ghv6nr DOI: https://doi.org/10.1016/S0021-9258(19)52451-6
Luna LG, editor. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York (USA): Mc Graw–Hill. 1968. 258 p.
Gonzalez F, Sia CL, Shepard MK, Rote NS, Minium J. Hyperglycemia–induced oxidative stress is independent of excess abdominal adiposity in normal–weight women with polycystic ovary syndrome. Hum. Reprod. [Internet]. 2012; 27(12):3560–3568. doi: https://doi.org/pfx9 DOI: https://doi.org/10.1093/humrep/des320
Sandhu JK, Waqar A, Jain A, Joseph C, Srivastava K, Ochuba O, Alkayyali T, Rua SW, Poudel S. Oxidative stress in polycystic ovarian syndrome and the effect of antioxidant N–acetylcysteine on ovulation and pregnancy rate. Cureus [Internet]. 2021; 13(9):e17887. doi: https://doi.org/pfzb DOI: https://doi.org/10.7759/cureus.17887
Bannigida DM, Nayak BS, Vijayaraghavan R. Insulin resistance and oxidative marker in women with PCOS. Arch. Physiol. Biochem. [Internet]. 2020; 126(2):183–186. doi: https://doi.org/gpsbrj DOI: https://doi.org/10.1080/13813455.2018.1499120
Cheng X, He B. Clinical and biochemical potential of antioxidants in treating polycystic ovary syndrome. Int. J. Womens Health. [Internet]. 2022; 14:467–479. doi: https://doi.org/pfzc DOI: https://doi.org/10.2147/IJWH.S345853
Murri M, Luque–Ramírez M, Insenser M, Ojeda–Ojeda M, Escobar–Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta–analysis. Hum. Reprod. Update. [Internet]. 2013; 19(3):268–288. doi: https://doi.org/f4v8r5 DOI: https://doi.org/10.1093/humupd/dms059
Zeber–Lubecka N, Ciebiera M, Hennig EE. Polycystic ovary syndrome and oxidative stress—from bench to bedside. Int. J. Mol. Sci. [Internet]. 2023; 24(18):14126. doi: https://doi.org/g87tq9 DOI: https://doi.org/10.3390/ijms241814126
Uçkan K, Demir H, Başkıran Y, Demir C. Investigation of activities enzyme prolidase (pro) and glutathione s–transferase (gst) in polycystic ovary syndrome (pcos) patients. J. Sci. Rep.–A. [Internet]. 2022 [cited 12 Dec. 2024]; (50):20–31. Available in: https://goo.su/Vuoz0Ic
Ghowsi M, Khazali H, Sisakhtnezhad S. The effect of resveratrol on oxidative stress in the liver and serum of a rat model of polycystic ovary syndrome: An experimental study. Int. J. Reprod. Biomed. [Internet]. 2018; 16(3):149. PMID: 29766146. Available in: https://n9.cl/ckewf DOI: https://doi.org/10.29252/ijrm.16.3.149
Rojas J, Chávez M, Olivar L, Rojas M, Morillo J, Mejías J, Calvo M, Bermúdez, V. Polycystic ovary syndrome, insulin resistance, and obesity: Navigating the pathophysiologic labyrinth. Int. J. Reprod. Med. [Internet]. 2014; 2014(1):719050. doi: https://doi.org/gcbgsm DOI: https://doi.org/10.1155/2014/719050
Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil. Steril. [Internet]. 2003; 80(1):123–127. doi: https://doi.org/fjb53t DOI: https://doi.org/10.1016/S0015-0282(03)00571-5
Moghetti P. Insulin resistance and polycystic ovary syndrome. Curr. Pharm. Des. [Internet]. 2016; 22(36):5526– 5534. doi: https://doi.org/f9cjsn DOI: https://doi.org/10.2174/1381612822666160720155855
Pandey V, Singh A, Singh A, Krishna, A, Pandey U, Tripathi YB. Role of oxidative stress and low–grade inflammation in letrozole–induced polycystic ovary syndrome in the rat. Reprod. Biol. [Internet]. 2016; 16(1):70–77. doi: https://doi.org/pfzf DOI: https://doi.org/10.1016/j.repbio.2015.12.005
Jahan S, Abid A, Khalid S, Afsar T, Ain QU, Shaheen G, Almajwal A, Razak S. Therapeutic potentials of Quercetin in management of polycystic ovarian syndrome using Letrozole induced rat model: a histological and a biochemical study. J. Ovarian Res. [Internet]. 2018; 11:1–10. doi: https://doi.org/gqpv5b DOI: https://doi.org/10.1186/s13048-018-0400-5
Reddy PS, Begum N, Mutha S, Bakshi V. Beneficial effect of Curcumin in Letrozole induced polycystic ovary syndrome. Asian Pacif. J. Reprod. [Internet]. 2016; 5(2):116–122. doi: https://doi.org/ghhdzb DOI: https://doi.org/10.1016/j.apjr.2016.01.006
Hassan RJ, Al–Husseini AMH. Estimation of catalase activity and Malondialdehyde levels in blood groups ABO of PCOS patients. J. Physics: Con. Ser. [Internet]. 2019; 1294(6):062100. doi: https://doi.org/pfzg DOI: https://doi.org/10.1088/1742-6596/1294/6/062100
Huang Y, Zhang X. Luteolin alleviates polycystic ovary syndrome in rats by resolving insulin resistance and oxidative stress. Am. J. Physiol. Endocrinol. Metab. [Internet]. 2021; 320(6):1085–1092. doi: https://doi.org/gq6pdc DOI: https://doi.org/10.1152/ajpendo.00034.2021
Kaya E, Yılmaz S, Ceribasi S. Protective role of propolis on low and high dose furan–induced hepatotoxicity and oxidative stress in rats. J. Vet. Res. [Internet]. 2019; 63(3):423–431. doi: https://doi.org/mhzs DOI: https://doi.org/10.2478/jvetres-2019-0054
Yang J, Chen C. Hormonal changes in PCOS. J. Endocrinol. [Internet]. 2024; 261(1):e230342. doi: https://doi.org/pfzh DOI: https://doi.org/10.1530/JOE-23-0342
Gozukara I, Dokuyucu R, Özgür T, Özcan O, Pınar N, Kurt RK, Kucur SK, Dolapcioglu, K. Histopathologic and metabolic effect of ursodeoxycholic acid treatment on PCOS rat model. Gynecol. Endocrinol. [Internet]. 2016; 32(6):492–497. doi: https://doi.org/pfzj DOI: https://doi.org/10.3109/09513590.2015.1134478
Gonzales GF, Cordova A, Gonzales C, Chung A, Vega K, Villena A. Lepidium meyenii (Maca) improved semen parameters in adult men. Asian J. Androl. [Internet]. 2001; 3(4): 301–304. PMID: 11753476. Available in: https://n9.cl/vnm83
Lee MS, Shin BC, Yang EJ, Lim HJ, Ernst E. Maca (Lepidium meyenii) for treatment of menopausal symptoms: a systematic review. Maturitas. [Internet]. 2011; 70(3):227–233. doi: https://doi.org/frvqvc DOI: https://doi.org/10.1016/j.maturitas.2011.07.017
Dording CM, Schettler PJ, Dalton ED, Parkin SR, Walker RS, Fehling KB, Fava M, Mischoulon D. A double–blind placebo– controlled trial of maca root as treatment for antidepressant– induced sexual dysfunction in women. Evid. Based Complement. Alternat. Med. [Internet]. 2015; 2015(1):949036. doi: https://doi.org/gb6bfd DOI: https://doi.org/10.1155/2015/949036
Bower–Cargill C, Yarandi N, Petróczi A. A systematic review of the versatile effects of the Peruvian Maca Root (Lepidium meyenii) on sexual dysfunction, menopausal symptoms and related conditions. Phytomed. Plus. [Internet]. 2022; 2(4):100326. doi: https://doi.org/pfzk DOI: https://doi.org/10.1016/j.phyplu.2022.100326
Ali–Amin A, Mohammed–Rabeh N, Saad–Obid A. The use of maca (Lepidium meyenii) to ımprove semen quality in diabetic obese male rats. Homo Econ. J. [Internet]. 2024; 40(4):1–16. doi: https://doi.org/pfzm DOI: https://doi.org/10.21608/jhe.2024.390715
Ai Z, Cheng AF, Yu YT, Yu LJ, Jin W. Antidepressant–like behavioral, anatomical, and biochemical effects of petroleum ether extract from maca (Lepidium meyenii) in mice exposed to chronic unpredictable mild stress. J. Med. Food. [Internet]. 2014; 17(5):535–542. doi: https://doi.org/f5355s DOI: https://doi.org/10.1089/jmf.2013.2950
Onaolapo AY, Oladipo BP, Onaolapo OJ. Cyclophosphamide– induced male subfertility in mice: An assessment of the potential benefits of Maca supplement. Andrologia. [Internet]. 2018; 50(3):e12911. doi: https://doi.org/pfzn DOI: https://doi.org/10.1111/and.12911
Qiu C, Zhu T, Lan L, Zeng Q, Du Z. Analysis of maceaene and macamide contents of petroleum ether extract of black, yellow, and purple Lepidium meyenii (Maca) and their antioxidant effect on diabetes mellitus rat model. Braz. Arch. Biol. Technol. [Internet]. 2016; 59:e16150462. doi: https://doi.org/f822bf DOI: https://doi.org/10.1590/1678-4324-2016150462
Oshima M, Gu Y, Tsukada S. Effects of Lepidium meyenii Walp and Jatropha macrantha on blood levels of estradiol–17 β, progesterone, testosterone and the rate of embryo implantation in mice. J. Vet. Med. Sci. [Internet]. 2003; 65(10):1145–1146. doi: https://doi.org/bxx32h DOI: https://doi.org/10.1292/jvms.65.1145
Ruiz–Luna AC, Salazar S, Aspajo NJ, Rubio J, Gasco M, Gonzales GF. Lepidium meyenii (Maca) increases litter size in normal adult female mice. Reprod. Biol. Endocrinol. [Internet]. 2005; 3:1–6. doi: https://doi.org/fckb67 DOI: https://doi.org/10.1186/1477-7827-3-16
Meissner HO, Mrozikiewicz P, Bobkiewicz–Kozlowska T, Mscisz A, Kedzia B, Lowicka A, Reich–Bilinska H, Kapczynski W, Barchia I. Hormone–balancing effect of pre–gelatinized organic Maca (Lepidium peruvianum Chacon):(I) biochemical and pharmacodynamic study on Maca using clinical laboratory model on ovariectomized rats. Int. J. Biomed. Sci. [Internet]. 2006; 2(3):260–272. Available in: https://n9.cl/h0l842 DOI: https://doi.org/10.59566/IJBS.2006.2260