Histopathological changes and endoplasmic reticulum stress in type 1 diabetic mouse lung tissue
Abstract
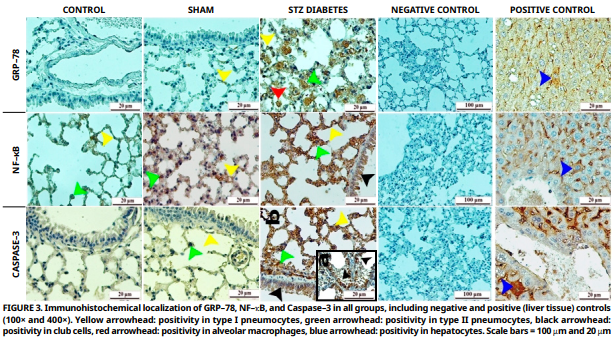
The aim of this study was to investigate the impact of type 1 diabetes on lung tissue using histopathological and immunohistochemical techniques, with a particular focus on whether it induces endoplasmic reticulum stress in the lung. The experiment utilized 18 male Balb/c mice, two months old with an average weight of 40 grams, randomly assigned to three groups: Control (n = 6), Sham (n = 6), and Streptozotocin diabetes group (n= 6). Diabetes was induced in the Streptozotocin group via a single intraperitoneal injection of 100 mg·kg-1 Streptozotocin. Following the experimental period, histopathological, immunohistochemical, and semi-quantitative analyses were conducted. Histopathological analysis revealed significant degeneration, apoptotic changes in epithelial cells and connective tissue, capillary dilation and inflammatory cell infiltration in the Streptozotocin group compared with the control and sham groups. Immunohistochemical analyses revealed a strong positive immunoreactivity for glucose regulated protein 78, nuclear factor kappa B and caspase–3 in type I and II pneumocytes, club cells, endothelial cells and alveolar macrophages in the Streptozotocin group. These observations imply the possible induction of endoplasmic reticulum stress in the lungs by type 1 diabetes and finally culminate into various histopathological alterations.
Downloads
References
Leslie KA, Lekka C, Richardson SJ, Russell MA, Morgan NG. Regulation of STAT1 signaling in human pancreatic β–Cells by the Lysine Deacetylase HDAC6: A new therapeutic opportunity in type 1 Diabetes? Diabetes [Internet]. 2024; 73(9):1473–1485. doi: https://doi.org/qkpk DOI: https://doi.org/10.2337/db24-0008
Abdel–Ghaffar A, Elhossary GG, Mahmoud AM, Elshazly AHM, Hassanin OA, Saleh A, Mansour SM, Metwally FG, Hanafy LK, Karam SH, Darweesh N, Ata AM. Effects of 4–phenylbutyric acid on the development of diabetic retinopathy in diabetic rats: regulation of endoplasmic reticulum stress–oxidative activation. Arch. Physiol. Biochem. [Internet]. 2023; 129(4):964–974. doi: https://doi.org/qkpm DOI: https://doi.org/10.1080/13813455.2021.1888302
Chen S, Novick P, Ferro–Novick S. ER structure and function. Curr. Opin. Cell Biol. [Internet]. 2013; 25(4):428–433. doi: https://doi.org/gjfw25 DOI: https://doi.org/10.1016/j.ceb.2013.02.006
Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N, Montibeller L, More S, Papaioannou A, Püschel F, Sassano ML, Skoko J, Agostinis P, de Belleroche J, Eriksson LA, Fulda S, Gorman AM, Healy S, Kozlov A, Muñoz–Pinedo C, Rehm M, Chevet E, Samali A. Endoplasmic reticulum stress signalling – from basic mechanisms to clinical applications. FEBS J. [Internet]. 2019; 286(2):241–278. doi: https://doi.org/gf4ch9 DOI: https://doi.org/10.1111/febs.14608
Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. [Internet]. 2007; 18(6):716–731. doi: https://doi.org/c3bstq DOI: https://doi.org/10.1016/j.semcdb.2007.09.003
Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr. Opin. Cell. Biol. [Internet]. 2011; 23(2):150–156. doi: https://doi.org/djz3pr DOI: https://doi.org/10.1016/j.ceb.2010.09.007
Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF–alpha–induced apoptosis by NF–kappaB. Science [Internet]. 1996; 274(5288):787–789. doi: https://doi.org/b5xc4s DOI: https://doi.org/10.1126/science.274.5288.787
Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, Tohyama M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase–3 via caspase–12. Neurosci. Lett. [Internet]. 2004; 357(2):127–130. doi: https://doi.org/dhfwt5 DOI: https://doi.org/10.1016/j.neulet.2003.12.080
Liu G, Sun Y, Li Z, Song T, Wang H, Zhang Y, Ge Z. Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem. Biophys. Res. Commun. [Internet]. 2008; 370(4):651–656. doi: https://doi.org/fr53z8 DOI: https://doi.org/10.1016/j.bbrc.2008.04.031
Zhao Y, Yan Y, Zhao Z, Li S, Yin J. The dynamic changes of endoplasmic reticulum stress pathway markers GRP78 and CHOP in the hippocampus of diabetic mice. Brain Res. Bull. [Internet]. 2015; 111:27–35. doi: https://doi.org/f626t2 DOI: https://doi.org/10.1016/j.brainresbull.2014.12.006
Galán M, Kassan M, Choi SK, Partyka M, Trebak M, Henrion D, Matrougui K. A novel role for epidermal growth factor receptor tyrosine kinase and its downstream endoplasmic reticulum stress in cardiac damage and microvascular dysfunction in type 1 diabetes mellitus. Hypertension [Internet]. 2012; 60(1):71–80. doi: https://doi.org/qkpn DOI: https://doi.org/10.1161/HYPERTENSIONAHA.112.192500
Teodoro JS, Nunes S, Rolo AP, Reis F, Palmeira CM. Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of Diabetes. Front. Physiol. [Internet]. 2019; 9:1857. doi: https://doi.org/qkpp DOI: https://doi.org/10.3389/fphys.2018.01857
Tanjore H, Blackwell TS, Lawson WE. Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. [Internet]. 2012; 302(8):L721–729. doi: https://doi.org/fxr7zc DOI: https://doi.org/10.1152/ajplung.00410.2011
Kida K, Utsuyama M, Takizawa T, Thurlbeck WM. Changes in lung morphologic features and elasticity caused by streptozotocin–induced diabetes mellitus in growing rats. Am. Rev. Respir. Dis. [Internet]. 1983; 128(1):125–131. doi: https://doi.org/qkpq DOI: https://doi.org/10.1164/arrd.1983.128.1.125
Popov D, Simionescu M. Alterations of lung structure in experimental diabetes, and diabetes associated with hyperlipidaemia in hamsters. Eur. Respir. J. [Internet]. 1997; 10(8):1850–1858. doi: https://doi.org/d27qg3 DOI: https://doi.org/10.1183/09031936.97.10081850
Elderbi M, Omar A, Omar H, Elburi A, Ibrahim M. Consequences of alloxan–induced diabetes on hematological and hepatic parameters in albino mice. Khalij–Libya J. Dent. Med. Res. [Internet]. 2025; 9(1)38–43.doi: https://doi.org/qkpr DOI: https://doi.org/10.47705/kjdmr.25911006
Deprem T, Yıldız SE, Sari EK, Bingol SA, Tasci SK, Aslan S, Sozmen M, Nur G. Distribution of glutathione peroxidase 1 in liver tissues of healthy and diabetic rats treated with capsaisin. Biotech. Histochem. [Internet]. 2015; 90(1):1–7. doi: https://doi.org/qkps DOI: https://doi.org/10.3109/10520295.2014.919024
Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. [Internet]. 2008; 2008:pdb.prot4986. doi: https://doi.org/cq3zx8 DOI: https://doi.org/10.1101/pdb.prot4986
Bancroft J, Cook H. Manual of Histological Techniques. New York (USA): Churchill Livingstone; 1984.
Hsu SM, Raine L, Fanger H. Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. [Internet]. 1981; 29(4):577–580. doi: https://doi.org/b573dv DOI: https://doi.org/10.1177/29.4.6166661
Shu SY, Ju G, Fan LZ. The glucose oxidase–DAB–nickel method in peroxidase histochemistry of the nervous system. Neurosci. Lett. [Internet]. 1988; 85(2):169–171. doi: https://doi.org/bfvmmc DOI: https://doi.org/10.1016/0304-3940(88)90346-1
Okihiro MS, Hinton DE. Partial hepatectomy and bile duct ligation in rainbow trout (Oncorhynchus mykiss): histologic, immunohistochemical and enzyme histochemical characterization of hepatic regeneration and biliary hyperplasia. Toxicol. Pathol. [Internet]. 2000; 28(2):342–356. doi: https://doi.org/bdh4pf DOI: https://doi.org/10.1177/019262330002800215
Bencze J, Szarka M, Kóti B, Seo W, Hortobágyi TG, Bencs V, Módis LV, Hortobágyi T. Comparison of semi–quantitative scoring and artificial intelligence aided digital image analysis of chromogenic immunohistochemistry. Biomolecules [Internet]. 2021; 12(1):19. doi: https://doi.org/gpfs9p DOI: https://doi.org/10.3390/biom12010019
Wang D, Ma Y, Tong X, Zhang Y, Fan H. Diabetes mellitus contributes to idiopathic pulmonary fibrosis: A review from clinical appearance to possible pathogenesis. Front. Public. Health. [Internet]. 2020; 8:196. doi: https://doi.org/gp78v9 DOI: https://doi.org/10.3389/fpubh.2020.00196
Hu Y, Ma Z, Guo Z, Zhao F, Wang Y, Cai L, Yang J. Retraction Note to: Type 1 diabetes mellitus is an independent risk factor for pulmonary fibrosis. Cell Biochem. Biophys. [Internet]. 2023; 81(2):377. doi: https://doi.org/qkpt DOI: https://doi.org/10.1007/s12013-023-01127-2
Tiengo A, Fadini GP, Avogaro A. The metabolic syndrome, diabetes and lung dysfunction. Diabetes Metab. [Internet]. 2008; 34(5):447–454. doi: https://doi.org/c6fp8w DOI: https://doi.org/10.1016/j.diabet.2008.08.001
Lam TYW, Nguyen N, Peh HY, Shanmugasundaram M, Chandna R, Tee JH, Ong CB, Hossain MZ, Venugopal S, Zhang T, Xu S, Qiu T, Kong WT, Chakarov S, Srivastava S, Liao W, Kim JS, Teh M, Ginhoux F, Fred–Wong WS, Ge R. ISM1 protects lung homeostasis via cell–surface GRP78–mediated alveolar macrophage apoptosis. Proc. Natl. Acad. Sci. USA. [Internet]. 2022; 119(4):e2019161119. doi: https://doi.org/gqkmq6 DOI: https://doi.org/10.1073/pnas.2019161119
Kropski JA, Blackwell TS. Endoplasmic reticulum stress in the pathogenesis of fibrotic disease. J. Clin. Invest. [Internet]. 2018; 128(1):64–73. doi: https://doi.org/gcrx8s DOI: https://doi.org/10.1172/JCI93560
Chipurupalli S, Samavedam U, Robinson N. Crosstalk Between ER Stress, Autophagy and Inflammation. Front. Med. [Internet]. 2021; 8:758311. doi: https://doi.org/gpjnqx DOI: https://doi.org/10.3389/fmed.2021.758311
Patel S, Santani D. Role of NF–kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep. [Internet]. 2009; 61(4):595–603. doi: https://doi.org/gh6vh4 DOI: https://doi.org/10.1016/S1734-1140(09)70111-2
Raguraman R, Srivastava A, Munshi A, Ramesh R. Therapeutic approaches targeting molecular signaling pathways common to diabetes, lung diseases and cancer. Adv. Drug. Deliv. Rev. [Internet]. 2021; 178:113918. doi: https://doi.org/gprtxn DOI: https://doi.org/10.1016/j.addr.2021.113918
Chaudhari N, Talwar P, Parimisetty A, Lefebvre–d’Hellencourt C, Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. [Internet]. 2014; 8:213. doi: https://doi.org/gpvmbj DOI: https://doi.org/10.3389/fncel.2014.00213
Radhakrishnan SK, Kamalakaran S. Pro–apoptotic role of NF– kappaB: implications for cancer therapy. Biochim. Biophys. Acta (BBA). [Internet]. 2006; 1766(1):53–62. doi: https://doi.org/b2zjcv DOI: https://doi.org/10.1016/j.bbcan.2006.02.001
Zheng H, Wu J, Jin Z, Yan LJ. Potential biochemical mechanisms of lung injury in Diabetes. Aging Dis. [Internet]. 2017; 8(1):7–16. doi: https://doi.org/qkpv DOI: https://doi.org/10.14336/AD.2016.0627
Kucharczak J, Simmons MJ, Fan Y, Gélinas C. To be, or not to be: NF–κB is the answer—role of Rel/NF–κB in the regulation of apoptosis. Oncogene [Internet]. 2003; 22(56):8961–8982. doi: https://doi.org/dq49ph DOI: https://doi.org/10.1038/sj.onc.1207230