Effects of Lactobacillus rhamnosus GG on some adipokine levels in rats fed Western diet
Abstract
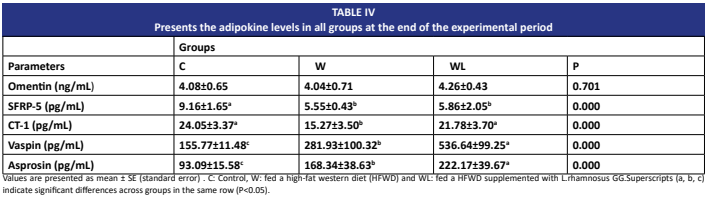
Adipokines regulate energy homeostasis. High-fat Western diet leads to obesity and disrupts adipocyte metabolism. Probiotics such as Lactobacillus rhamnosus GG are effective in obesity management. This study investigates the effect of Lactobacillus rhamnosus GG on serum adipokine levels in rats fed a High-fat Western diet. Thirty 5-6 week old male Wistar albino rats (Rattus norvegicus domestica) were randomly selected and divided into three groups: control (standard chow), W (45% fat), and a WLgroup supplemented orally with Lactobacillus rhamnosus GG in vegetable oil for 16 weeks. At the end of the study, blood samples were collected from rats via cardiac puncture, and serum adipokine levels were measured using ELISA kits. Body weight, naso-anal length, and BMI (body mass index) exhibited statistically significant increases in the W group. In the Lactobacillus rhamnosus GG-supplemented group (WL), body weight and BMI decreased, while naso-anal length increased. SFRP5 and CT1 levels were low, and asprosin and vaspin levels were high in the Wgroup. CT1, vaspin, and asprosin levels increased in the Lactobacillus rhamnosus GG- supplemented group. In conclusion, Lactobacillus rhamnosus GG alleviates obesity, increases naso-anal length, and reduces BMI in rats fed a High-fat Western diet. These results suggest that L. rhamnosus may regulate serum CT1, vaspin, and asprosin levels, but not omentin or SFRP5 in the High-fat Western diet model.
Downloads
References
Bortolin RC, Vargas AR, Gasparotto J, Chaves PR, Schnorr CE, Martinello KB, Silveira AK, Rabelo TK, Gelain DP, Moreira JCF. A new animal diet based on human Western diet is a robust diet-induced obesity model: comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int. J. Obes. [Internet]. 2018; 42(3):525-534. doi: https://doi.org/gdbp39 DOI: https://doi.org/10.1038/ijo.2017.225
Vileigas DF, de Souza-Borges SL, Corrêa CR, Silva CCVA, de Campos DHS, Padovani CR, Cicogna AC. The effects of two types of Western diet on the induction of metabolic syndrome and cardiac remodeling in obese rats. J. Nutr. Biochem. [Internet]. 2021; 92:108625. doi: https://doi.org/p8s4 DOI: https://doi.org/10.1016/j.jnutbio.2021.108625
Lynes MD, Tseng YH. Deciphering adipose tissue heterogeneity. Ann. NY. Acad. Sci. [Internet]. 2018; 1411(1):5-20. doi: https://doi.org/p8s5 DOI: https://doi.org/10.1111/nyas.13398
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. [Internet]. 1994; 372(6505):425-432. doi: https://doi.org/cjkx2m DOI: https://doi.org/10.1038/372425a0
Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin. Appl. [Internet]. 2012; 6(1-2):91-101. doi: https://doi.org/c58stx DOI: https://doi.org/10.1002/prca.201100052
Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. [Internet]. 2016; 5:47-56. doi: https://doi.org/gf5rt5 DOI: https://doi.org/10.2147/ITT.S73223
Sagkan-Ozturk A, Arpaci A. The comparison of changes in fecal and mucosal microbiome in metabolic endotoxemia induced by a high-fat diet. Anaerobe. [Internet]. 2022; 77:102615. doi: https://doi.org/p8s6 DOI: https://doi.org/10.1016/j.anaerobe.2022.102615
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid. Res. [Internet]. 2013; 54(9):2325-2340. doi: https://doi.org/f5ct3p DOI: https://doi.org/10.1194/jlr.R036012
Martinez KB, Leone V, Chang EB. Western diets, gut dysbiosis, and metabolic diseases: Are they linked?. Gut Microbes. [Internet]. 2017; 8(2):130-142. doi: https://doi.org/p8s7 DOI: https://doi.org/10.1080/19490976.2016.1270811
Janiszewska J, Ostrowska J, Szostak-Węgierek D. The influence of nutrition on adiponectin—A narrative review. Nutrients. [Internet]. 2021; 13(5):1394. doi: https://doi.org/p8s8 DOI: https://doi.org/10.3390/nu13051394
Sahin-Efe A, Katsikeris F, Mantzoros CS. Advances in adipokines. Metabolism. [Internet]. 2012; 61(12):1659–1665. doi: https://doi.org/gpqmrz DOI: https://doi.org/10.1016/j.metabol.2012.09.001
Sanz Y, Rastmanesh R, Agostoni C. Understanding the role of gut microbes and probiotics in obesity: how far are we?. Pharmacol. Res. [Internet]. 2013; 69(1):144–155. doi: https://doi.org/f4qp74 DOI: https://doi.org/10.1016/j.phrs.2012.10.021
Wu T, Sun M, Liu R, Sui W, Zhang J, Yin J, Fang S, Zhu J, Zhang M. Bifidobacterium longum subsp. longum Remodeled Roseburia and Phosphatidylserine Levels and Ameliorated Intestinal Disorders and liver Metabolic Abnormalities Induced by High-Fat Diet. J. Agric. Food Chem. [Internet]. 2020; 68(16):4632–4640. doi: https://doi.org/p8t5 DOI: https://doi.org/10.1021/acs.jafc.0c00717
Li H, Liu F, Lu J, Shi J, Guan J, Yan F, Li B, Huo G. Probiotic Mixture of Lactobacillus plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front. Microbiol. [Internet]. 2020; 11:512. doi: https://doi.org/p8t6 DOI: https://doi.org/10.3389/fmicb.2020.00512
Guo Y , Wang Z , Chen L , Tang L , Wen S , Liu Y , Yuan J . Diet induced maternal obesity affects offspring gut microbiota and persists into young adulthood. Food Funct. [Internet]. 2018; 9(8):4317-4327. doi: https://doi.org/p8t7 DOI: https://doi.org/10.1039/C8FO00444G
Bubnov R, Babenko L, Lazarenko L, Kryvtsova M, Shcherbakov O, Zholobak N, Golubnitschaja O, Spivak M. Can tailored nanoceria act as a prebiotic? Report on improved lipid profile and gut microbiota in obese mice. EPMA J. [Internet]. 2019; 10(4):317-335. doi: https://doi.org/gknzct DOI: https://doi.org/10.1007/s13167-019-00190-1
Sun M, Wu T, Zhang G, Liu R, Sui W, Zhang M, Geng J, Yin J, Zhang M. Lactobacillus rhamnosus LRa05 improves lipid accumulation in mice fed with a high fat diet via regulating the intestinal microbiota, reducing glucose content and promoting liver carbohydrate metabolism. Food Funct. [Internet]. 2020; 11(11):9514-9525. doi: https://doi.org/p8t8 DOI: https://doi.org/10.1039/D0FO01720E
López-Almada G, Mejía-León ME, Salazar-López NJ. Probiotic, Postbiotic, and Paraprobiotic Effects of Lactobacillus rhamnosus as a Modulator of Obesity-Associated Factors. Foods. [Internet]. 2024; 13(22):3529. doi: https://doi.org/p8t9 DOI: https://doi.org/10.3390/foods13223529
Demir EA, Gulbol-Duran G, Urhan-Kucuk M, Dogan H, Tutuk O, Cimen F, Bayirli M, Tumer C, Duran N. Behavioral and Cognitive Consequences of Obesity in Parents and Offspring in Female and Male Rats: Implications of Neuroinflammation and Neuromodulation. Mol. Neurobiol. [Internet]. 2022; 59(6):3947-3968. doi: https://doi.org/p8vb DOI: https://doi.org/10.1007/s12035-022-02831-5
Yung-Tsung C, Shiou-Yun C, Ai-Hua H, Yu-Chun L, Jin-Seng L. Lactobacillus rhamnosus Strain LRH05 Intervention Ameliorated Body Weight Gain and Adipose Inflammation via Modulating the Gut Microbiota in High-Fat Diet-Induced Obese Mice. Mol. Nutr. Food Res. [Internet]. 2022; 66(1):e2100348. doi: https://doi.org/gnj7fb DOI: https://doi.org/10.1002/mnfr.202100348
Alipour H, Gazerani P, Heidari M, Dardmeh F. Modulatory Effect of Probiotic Lactobacillus rhamnosus PB01 on Mechanical Sensitivity in a Female Diet-Induced Obesity Model. Pain Res Manag. [Internet]. 2021; 2021:5563959. doi: https://doi.org/p8vc DOI: https://doi.org/10.1155/2021/5563959
Khanna S, Walia S, Kondepudi KK,Shukla G. Administration of indigenous probiotics modulate high-fat diet-induced metabolic syndrome in Sprague Dawley rats. Antonie Van Leeuwenhoek. [Internet]. 2020; 113(9):1345–1359. doi: https://doi.org/p8vd DOI: https://doi.org/10.1007/s10482-020-01445-y
Borgeraas H, Johnson LK, Skattebu J, Hertel JK, Hjelmesaeth J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. [Internet]. 2018; 19(2):219–32. doi: https://doi.org/gm8c7q DOI: https://doi.org/10.1111/obr.12626
Onubi OJ, Poobalan AS, Dineen B, Marais D, McNeill G. Effects of probiotics on child growth: a systematic review. J. Health Popul. Nutr. [Internet]. 2015; 34:8. doi: https://doi.org/gn6j9h DOI: https://doi.org/10.1186/s41043-015-0010-4
Jensen EA, Young JA, Jackson Z, Busken J, List EO, Carroll RK, Kopchick JJ, Murphy ER, Berryman DE. Growth Hormone Deficiency and Excess Alter the Gut Microbiome in Adult Male Mice. Endocrinology. [Internet]. 2020; 161(4):bqaa026. doi: https://doi.org/p8vf DOI: https://doi.org/10.1210/endocr/bqaa026
Tyagi AM, Yu M, Darby TM, Vaccaro C, Li JY, Owens JA, Hsu E, Adams J, Weitzmann MN, Jones RM, Pacifici R. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity. [Internet]. 2018; 49(6):1116-1131.e7. doi: https://doi.org/gg4khx DOI: https://doi.org/10.1016/j.immuni.2018.10.013
Shi J, Zhao G, Huang X, Li X, Ma Y, Yang K. Effects of Lactobacillus rhamnosus Supplementation on Growth Performance, Immune Function, and Antioxidant Capacity of Newborn Foals. J. Equine Vet. Sci. [Internet]. 2023; 129:104501. doi: https://doi.org/p8vg DOI: https://doi.org/10.1016/j.jevs.2023.104501
Kang T, Ree J, Park JW, Choe H, Park YI. Anti-Obesity Effects of SPY Fermented with Lactobacillus rhamnosus BST-L.601 via Suppression of Adipogenesis and Lipogenesis in High-Fat Diet-Induced Obese Mice. Foods. [Internet]. 2023; 12(11):2202. doi: https://doi.org/p8xr DOI: https://doi.org/10.3390/foods12112202
Lee MW, Lee M, Oh KJ. Adipose Tissue-Derived Signatures for Obesity and Type 2 Diabetes: Adipokines, Batokines and MicroRNAs. J. Clin. Med. [Internet]. 2019; 8(6):854. doi: https://doi.org/ggzfmw DOI: https://doi.org/10.3390/jcm8060854
Nosrati-Oskouie M, Asghari G, Yuzbashian E, Aghili-Moghaddam NS, Zarkesh M, Safarian M, Mirmiran P. Does Dietary Intake Impact Omentin Gene Expression and Plasma Concentration? A Systematic Review. Lifestyle Genom. [Internet]. 2021; 14(2):49-61. doi: https://doi.org/g8vpgv DOI: https://doi.org/10.1159/000513885
Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Walsh K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. [Internet]. 2010; 329(5990):454–457. doi: https://doi.org/cskh6m DOI: https://doi.org/10.1126/science.1188280
Hu Z, Deng H, Qu H. Plasma SFRP5 levels are decreased in Chinese subjects with obesity and type 2 diabetes and negatively correlated with parameters of insulin resistance. Diabetes Res. Clin. Pract. [Internet]. 2013; 99(3):391-395. doi: https://doi.org/f2jt6d DOI: https://doi.org/10.1016/j.diabres.2012.11.026
Hu W, Li L, Yang M, Luo X, Ran W, Liu D, Xiong Z, Liu H, Yang G. Circulating Sfrp5 is a signature of obesityrelated metabolic disorders and is regulated by glucose and liraglutide in humans. J. Clin. Endocrinol. Metab. [Internet]. 2013; 98(1):290-298. doi: https://doi.org/f4hjh8 DOI: https://doi.org/10.1210/jc.2012-2466
Schulte DM, Müller N, Neumann K, Oberhäuser F, Faust M, Güdelhöfer H, Brandt B, Krone W, Laudes M. Proinflammatory wnt5a and anti-inflammatory sFRP5 are differentially regulated by nutritional factors in obese human subjects. PLoS One. [Internet]. 2012; 7(2):e32437. doi: https://doi.org/p8vh DOI: https://doi.org/10.1371/journal.pone.0032437
Tan X, Wang X, Chu H, Liu H, Yi X, Xiao Y. SFRP5 correlates with obesity and metabolic syndrome and increases after weight loss in children. Clin. Endocrinol. [Internet]. 2014; 81(3):363-369. doi: https://doi.org/f6c28b DOI: https://doi.org/10.1111/cen.12361
Sanchez-Infantes D, White UA, Elks CM, Morrison RF, Gimble JM, Considine RV, Ferrante AW, Ravussin E, Stephens JM. Oncostatin m is produced in adipose tissue and is regulated in conditions of obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. [Internet]. 2014; 99(2):E217-E225. doi: https://doi.org/f5vn8t DOI: https://doi.org/10.1210/jc.2013-3555
Moreno-Aliaga MJ, Pérez-Echarri N, Marcos-Gómez B, Larequi E, Gil-Bea FJ, Viollet B, Gimenez I, Martínez JA, Prieto J, Bustos M. Cardiotrophin-1 is a key regulator of glucose and lipid metabolism. Cell Metab. [Internet]. 2011; 14(2):242-253. doi: https://doi.org/cbjxhk DOI: https://doi.org/10.1016/j.cmet.2011.05.013
Hung HC, Lu FH, Wu HT, Ou HY, Yang YC, Wu JS, Chang CJ. Cardiotrophin-1 is inversely associated with obesity in non-diabetic individuals. Sci. Rep. [Internet]. 2015; 5:17438. doi: https://doi.org/f7zs4d DOI: https://doi.org/10.1038/srep17438
Feng Y, Ye D, Wang Z, Pan H, Lu X, Wang M, Xu Y, Yu J, Zhang J, Zhao M, Xu S, Pan W, Yin Z, Ye J, Wan J. The Role of Interleukin-6 Family Members in Cardiovascular Diseases. Front. Cardiovasc. Med. [Internet]. 2022; 9:818890. doi: https://doi.org/g6kvq6 DOI: https://doi.org/10.3389/fcvm.2022.818890
Moreno-Aliaga MJ, Romero-Lozano MA, Castaño D, Prieto J, Bustos M. Role of cardiotrophin-1 in obesity and insulin resistance. Adipocyte. [Internet]. 2012; 1(2):112-115. doi: https://doi.org/gb9wp3 DOI: https://doi.org/10.4161/adip.19696
Feng R, Li Y, Wang C, Luo C, Liu L, Chuo F, Li Q, Sun C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: a meta-analysis. Diabetes Res. Clin. Pract. [Internet]. 2014; 106(1):88-94. doi: https://doi.org/f2t82r DOI: https://doi.org/10.1016/j.diabres.2014.07.026
Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, Xia F, Saha PK, Del Solar M, Zhu B, York B, Sarkar P, Rendon DA, Gaber MW, LeMaire SA, Coselli JS, Milewicz DM, Sutton VR, Butte NF, Moore DD, Chopra AR. Asprosin, a Fasting-Induced Glucogenic Protein Hormone. Cell. [Internet]. 2016; 165(3):566-579. doi: https://doi.org/bd8m DOI: https://doi.org/10.1016/j.cell.2016.02.063
Abdel-Fadeil MR, Abd Allah ESH, Iraqy HM, Elgamal DA, Abdel-Ghani MA. Experimental obesity and diabetes reduce male fertility: Potential involvement of hypothalamic Kiss-1, pituitary nitric oxide, serum vaspin and visfatin. Pathophysiology. [Internet]. 2019; 26(3-4):181–189. doi: https://doi.org/p8vn DOI: https://doi.org/10.1016/j.pathophys.2019.02.001
Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, Shikata K, Hourai S, Futami J, Watanabe E, Matsuki Y, Hiramatsu R, Akagi S, Makino H, Kanwar YS. Visceral adipose tissue- derived serine protease inhibitor: a unique insulin- sensitizing adipocytokine in obesity. Proc Natl. Acad. Sci. U.S.A. [Internet]. 2005; 102(30):10610-10615. doi: https://doi.org/bsv426 DOI: https://doi.org/10.1073/pnas.0504703102
Corica D, Pepe G, Aversa T, Currò M, Curatola S, Li Pomi A, Alibrandi A, Ientile R, Wasniewska M. Meal- Related Asprosin Serum Levels Are Affected by Insulin Resistance and Impaired Fasting Glucose in Children With Obesity. Front. Endocrinol. (Lausanne). [Internet]. 2022; 12:805700. doi: https://doi.org/p8vq DOI: https://doi.org/10.3389/fendo.2021.805700
Klöting N, Kovacs P, Kern M, Heiker JT, Fasshauer M, Schön MR, Stumvoll M, Beck-Sickinger AG, Blüher M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia. [Internet]. 2011; 54(7):1819-1823. doi: https://doi.org/cpfkmf DOI: https://doi.org/10.1007/s00125-011-2137-1
Duerrschmid C, He Y, Wang C, Li C, Bournat JC, Romere C, Saha PK, Lee ME, Phillips KJ, Jain M, Jia P, Zhao Z, Farias M, Wu Q, Milewicz DM, Sutton VR, Moore DD, Butte NF, Krashes MJ, Xu Y, Chopra AR. Asprosin is a centrally acting orexigenic hormone. Nat. Med. [Internet]. 2017; 23(12):1444-1453. doi: https://doi.org/gcjq93 DOI: https://doi.org/10.1038/nm.4432
Gökdemir GS, Gökdemir MT, Tasdemir E, Yokus B, Baylan M. Importance of curcumin effect and asprosin level on glucose metabolism in diabetic rats. Med. Sci. [Internet]. 2023; 12(1):167-174. doi: https://doi.org/p8vr DOI: https://doi.org/10.5455/medscience.2022.12.281
Ugur K, Aydin S. Saliva and blood asprosin hormone concentration associated with obesity. Int. J. Endocrinol. [internet]. 2019; 2019:2521096. doi: https://doi.org/p8vs DOI: https://doi.org/10.1155/2019/2521096
Jeong E, Youn BS, Kim DW, Kim EH, Park JW, Namkoong C, Jeong JY, Yoon SY, Park JY, Lee KU, Kim MS. Circadian rhythm of serum vaspin in healthy male volunteers: relation to meals. J. Clin. Endocrinol. Metab. [Internet]. 2010; 95(4):1869-1875. doi: https://doi.org/d5p23w DOI: https://doi.org/10.1210/jc.2009-1088