Effects of orally administration free and Liposomal Levamisole on hematological and biochemical parameters in Sheep
Abstract
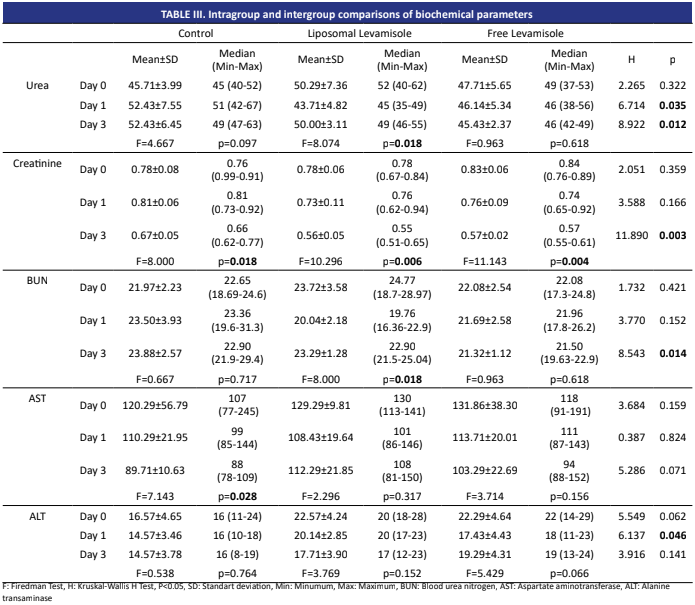
Analysis of haematological and biochemical parameters monitors animal health and guides diagnosis and treatment. This study compared the haematological and biochemical effects of free and liposomal levamisole given to sheep orally. The study included twenty-one female Curly breed sheep. The animals were divided into three groups: free levamisole (7.5 mg/kg), liposomal levamisole (7.5 mg/kg), and control (physiological serum 7.5 mL/kg). Blood samples were obtained on day (d) 0 (control), d 1, and d 3. Haematological parameters (WBC, RBC, HGB, HCT, MCV, MCH, MCHC, RDW-CV, RDW-SD, and PCT) were assessed utilizing a haematology analyzer, while biochemical parameters (urea, creatinine, AST, ALT, BUN) were evaluated using an autoanalyzer. On d 1, the liposome group exhibited the highest white blood cell measurements. Despite a reduction in PCT values on d 1 within the liposome group, an increase was observed again on d 3. Urea levels on d 1 and 3 were elevated in the control, liposomal levamisole, and free levamisole groups. On d 3, creatinine measurements indicated that levels in the control group were significantly elevated compared to those in the liposomal levamisole and free levamisole groups. On d 3, BUN measurements indicated that the mean for the control group was significantly elevated compared to the liposomal levamisole and free levamisole groups. The Neutrophil, Lymphocyte, and Monocyte counts in the liposomal and free levamisole groups of animals were significantly elevated compared to other measurements recorded on the d 3. This study’s findings demonstrate that liposomes affect haematological and biochemical parameters. The results demonstrate that liposomal levamisole causes no harmful effects on animals. It produces advantageous results. Further investigation is necessary to elucidate the effects of Liposomal Levamisole on hematological and biochemical parameters among various animal species.
Downloads
References
Sajid MS, Iqbal Z, Muhammad G, Iqbal MU. Immunomodulatory effect of various anti-parasitics: a review. Parasitol. [Internet]. 2006; 132(3):301-313. doi: https://doi.org/c2sx3r DOI: https://doi.org/10.1017/S0031182005009108
McKellar QA, Gokbulut C. Pharmacokinetic features of the antiparasitic macrocyclic lactones. Curr Pharm Biotechnol [Internet]. 2012[cited december 12 2024]; 13(6):888–911. Available in: https://doi.org/f3zc53 DOI: https://doi.org/10.2174/138920112800399194
Gholami MH, Rassouli A, Mirzaei S, Hashemi F. The potential immunomodulatory effect of levamisole in humans and farm animals. J. Adv. Vet. Anim. Res. [Internet]. 2023; 10(4):620-629. doi: https://doi.org/pcs9
Oliveira CC, Costa DFL, Limeira CH, Nogueira DB, Nascimento BHR, Vaz AFM. Anthelmintic intoxication in goats and sheep: A systematic review. Res. Vet. Sci. [Internet]. 2022; 152:657-662. doi: https://doi.org/pctb DOI: https://doi.org/10.1016/j.rvsc.2022.09.038
Baydan E. İmmünodepresant ve imunostimulantlar. Ankara Univ. Vet. Fak. Derg. [Internet]. 1995; 42(01):45-56. doi: https://doi.org/pctq
Petersen GH, Alzghari SK, Chee W, Sankari SS, La-Beck NM. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J. Control Release. [Internet]. 2016; 232:255-264. doi: https://doi.org/f8qt4w DOI: https://doi.org/10.1016/j.jconrel.2016.04.028
Ito K, Hamamichi S, Asano M, Hori Y, Matsui J, Iwata M, Funahashi Y, Umeda IO, Fujii H. Radiolabeled liposome imaging determines an indication for liposomal anticancer agent in ovarian cancer mouse xenograft models. Cancer Sci. [Internet]. 2016; 107(1):60-67. doi: https://doi.org/pctr DOI: https://doi.org/10.1111/cas.12841
Drulis-Kawa Z, Dorotkiewicz-Jach A. Liposomes as delivery systems for antibiotics. Int. J. Pharm. [Internet]. 2010; 387(1-2):187-198. doi: https://doi.org/cxmg8f DOI: https://doi.org/10.1016/j.ijpharm.2009.11.033
Jung SW, Thamphiwatana S, Zhang L, Obonyo M. Mechanism of antibacterial activity of liposomal linolenic acid against Helicobacter pylori. PLoS One. [Internet]. 2015;1 0(3):e0116519. doi: https://doi.org/gt9dtq DOI: https://doi.org/10.1371/journal.pone.0116519
Ghanbarzadeh S, Arami S. Enhanced transdermal delivery of diclofenac sodium via conventional liposomes, ethosomes, and transfersomes. Biomed. Res. Int. [Internet]. 2013; 2013(1):616810. doi: https://doi.org/f9vh5m DOI: https://doi.org/10.1155/2013/616810
Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. [Internet]. 2008; 5(4):487-495. doi: https://doi.org/fvsnn8 DOI: https://doi.org/10.1021/mp800032f
Szebeni J, Baranyi L, Savay S, Milosevits J, Bunger R, Laverman P, Metselaar JM, Storm G, Chanan-Khan A, Liebes L, Muggia FM, Cohen R, Barenholz Y, Alving CR. Role of complement activation in hypersensitivity reactions to doxil and hynic PEG liposomes: experimental and clinical studies. J. Liposome Res. [Internet]. 2002;12(1-2):165–72. doi: https://doi.org/c6mf6f DOI: https://doi.org/10.1081/LPR-120004790
Díaz-Martínez AE, Alcaide-Martín MJ, González-Gross M. Basal values of biochemical and hematological parameters in elite athletes. Int. J. Environ. Res. Public. Health. [Internet]. 2022;19(5):3059. doi: https://doi.org/pcts DOI: https://doi.org/10.3390/ijerph19053059
Pinto JM, Nogueira LS, Alves-Rios DR. Hematological parameters: is there a difference between those released by the hematological analyzer and to the customer?. Einstein. [Internet]. 2023; 21:eAO0501. doi: https://doi.org/pctt DOI: https://doi.org/10.31744/einstein_journal/2023AO0501
Gislefoss RE, Grimsrud TK, Mørkrid L. Stability of selected serum hormones and lipids after long-term storage in the Janus Serum Bank. Clin. Biochem. [Internet]. 2015; 48(6):364-369. doi: https://doi.org/f67nzk DOI: https://doi.org/10.1016/j.clinbiochem.2014.12.006
Kachhawa K, Kachhawa P, Varma M, Behera R, Agrawal D, Kumar S. Study of the stability of various biochemical analytes in samples stored at different predefined storage conditions at an Accredited Laboratory of India. J. Lab. Physicians. [Internet]. 2017; 9(1):11-15. doi: https://doi.org/f9kc5p DOI: https://doi.org/10.4103/0974-2727.187928
Susar H, Çelebi M, Çelebi Çağla, Çoban Özlem, Şen H, Karahan İzzet. Preparation and Characterisation of Liposomal Formulations of Levamisole and Albendazole Used in Veterinary Medicine. Rev. Cient. FCV-LUZ. [Internet]. 2024; 34(2):8. doi: https://doi.org/pctd DOI: https://doi.org/10.52973/rcfcv-e34401
Michael R. Sheldon MS, Michael J, Fillyaw W, Thompson D. The use and interpretation of the Friedman test in the analysis of ordinal-scale data in repeated measures designs. Physiother. Res. Int. [Internet]. 1996; 1(4):221-228. doi: https://doi.org/dchwsz DOI: https://doi.org/10.1002/pri.66
Frye EA, Behling-Kelly EL, Lejuene M, Webb JL. Complete blood count and biochemistry reference intervals for healthy adult sheep in the northeastern United States. Vet Clin Pathol. [Internet]. 2022; 51(1):119-25. doi: https://doi.org/g8gxtn DOI: https://doi.org/10.1111/vcp.13059
Rehni AK, Singh TG. Levamisole-induced reduction in seizure threshold: a possible role of nicotinic acetylcholine receptor-mediated pathway. Naunyn Schmiedebergs Arch. Pharmacol. [Internet]. 2010; 382(3):279-285. doi: https://doi.org/ftnpjb DOI: https://doi.org/10.1007/s00210-010-0543-4
Martin RJ, Robertson AP, Buxton SK, Beech RN, Charvet CL, Neveu C. Levamisole receptors: a second awakening. Trends Parasitol. [Internet]. 2012; 28(7):289-296. doi: https://doi.org/pctv DOI: https://doi.org/10.1016/j.pt.2012.04.003
Gokce HI, Gunes V, Erdogan HM, Citil M, Akca A, Yuksek N. The effects of levamisole poisoning on the haematological and biochemical parameters in dogs. Dtsch Tierarztl Wochenschr. [Internet]. 2004 [cited 13 Dec 2024]; 111(2):81-85. Available in: https://goo.su/DhUJtnT
Ravindra KJ, Bhikane UA, Ashwinin AB, Hidayat AS, Akash SJ. Acute oxyclozanide -levamisole poisoning in red kandhari bullocks. J. Adv. Vet. Res. [Internet]. 2016 [cited 13 Dec 2024]; 7(1):16-17. Available in: https://goo.su/o1gmlf
Müller KR, Dwyer C. Suspected levamisole intoxication in calves. N. Z. Vet. J. [Internet]. 2016; 64(4):257-260. doi: https://doi.org/pctp DOI: https://doi.org/10.1080/00480169.2016.1153438
Pandit S, Baidya S, Jas R. Effects of levamisole on haemato-biochemical profiles of naturally occurring gastrointestinal nematodosis in Garole sheep. IJRD. [Internet]. 2017 [cited 13 Dec 2024]; 2(1):1-6. Available in: https://goo.su/kY6KX
Yüksek N, Altuğ N, Gül A. Koyunlarda endoparazit enf eksiyonlarında triklabendazol - levamizol kombinasyonunun tedavi etkinliği. YYU. Vet. Fak. Derg. [Internet]. 2007 [cited Dec 13 2024]; 18(1):19-24. Available in: https://goo.su/ mThWGO DOI: https://doi.org/10.22201/fesz.20075502e.2023.13.52.88979
Atessahin A, Karahan I, Pirincci I. Effects of therapeutic and toxic doses of levamisole on thyroid hormones and some biochemical parameters in sheep. Cell Biochem. Funct. [Internet]. 2004; 22(5):281-286. doi: https://doi.org/brd99f DOI: https://doi.org/10.1002/cbf.1101
Bisalla M, Bi-Allah MB, Nnadiwa FO. Some haemato- biochemical parameters of sheep pre-treated with levamisole and transported by road. Agricultural J. [Internet]. 2011 [cited Dec 13, 2024]; 6(4):131-133. Available in: https://goo.su/ty9BR DOI: https://doi.org/10.3923/aj.2011.131.133
Asif MM, Zia-ur-Rahman, Naqvi ZH, Hassan MM, Nawaz A, Kausar F, Zakia F. Effect of levamisole administered orally on haematological and biochemical profiles of Sahiwal heifers. Vet. Arkhiv. [Internet]. 1995[cited 13 Dec 2024]; 65:185–192. Available in: https://goo.su/xmKu
Sandhu MA, Ahmad T. Haematological and serum biochemical profiles of buffalo heifers as influenced by levamisole. Comp. Clin. Path. [Internet]. 2003; 12(1):147–150. doi: https://doi.org/dgjd6f DOI: https://doi.org/10.1007/s00580-003-0490-2
Doğan E. Effects of levamisole application on immunity system in anthrax-vaccinated Cattle. KVJ. [Internet]. 2022; 15(3):348-354. doi: https://doi.org/pctw
Yuji-Sado R, de Almeida-Bicudo AJ, Possebon-Cyrino JE. Dietary levamisole influenced hematological parameters of juvenile pacu, Piaractus mesopotamicus (Holmberg 1887). JWAS. [Internet]. 2010; 41(S1):66-75. doi: https://doi.org/djnk74 DOI: https://doi.org/10.1111/j.1749-7345.2009.00334.x
Oliveira LCD, Brasiliense ARP, Dias MKR, Yoshioka ETO, Tavares-Dias M. Toxicological, hematological and immunological effects of levamisole and ivermectin diet supplementation on Colossoma macropomum (Serrasalmidae). Dis. Aquat Organ. [Internet]. 2019; 136(3):255-263. doi: https://doi.org/pctx DOI: https://doi.org/10.3354/dao03413
Kuropka P, Leśków A, Małolepsza-Jarmołowska K, Dobrzyński M, Tarnowska M, Majda J, Janeczek M, Zybura-Wszoła K, Gamian A. Effect of a single and triple dose of levamisole on hematological parameters in controlled inflammation model. Animals. [Internet]. 2022; 12(16):2110. doi: https://doi.org/pctz DOI: https://doi.org/10.3390/ani12162110
Shovon AK, Uddin N, Hridoy AF, Islam K, Miah MA. The effect of levamisole on growth performance, humoral immunity and blood biochemical profile in broiler chickens. J. Bangladesh Agril. Univ. [Internet]. 2020; 18(S1): 858–863. doi: https://doi.org/pct2 DOI: https://doi.org/10.5455/JBAU.13301