The effect of the pesticide Klaxon on the oxidative stress and the antioxidant responses of the Zebra mussel (Dreissena polymorpha)
Abstract
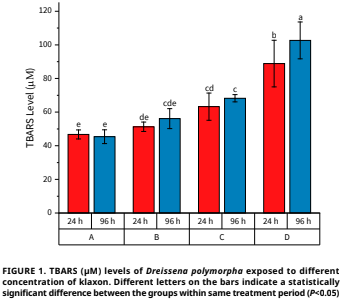
Klaxon pesticide represents a novel generation of insecticide employed in the control of diseases, pests and weeds in select agricultural regions. Pesticides that enter the aquatic environment indirectly have a detrimental impact on the organisms that inhabit this environment, and humans are ultimately exposed to these chemicals through the food chain. The present study investigated the toxicity of the klaxon pesticide in Dreissena polymorpha, a suitable bioindicator of water pollution, through the analysis of oxidative stress and metabolic biomarkers. The effects of klaxon at concentrations of 0.01, 0.15 and 0.30 mg·L-1 on oxidative stress and antioxidant changes in D. polymorpha were determined over a 24 – and 96–hour period. Enzyme–linked immunosorbent assay (ELISA) kits were employed to quantify the activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT) and thiobarbituric acid (TBARS), as well as to assess the levels of reduced glutathione (GSH). ELISA kits were used to determine results. Statistical evaluation of biomarker analyzes was performed using the SPSS 24.0 package program one–way ANOVA (Duncan 0.05) test. Significant decreases in GSH levels (P<0.05); significant increases in TBARS levels (P<0.05) were observed. Significant decreases (P<0.05) in SOD, CAT and GPx activities were observed. Considering the study data, it was determined that the klaxon pesticide penetrating the body of the living organism caused oxidative stress and changes in enzyme activities in D. polymorpha.
Downloads
References
İbadullayeva J, Jumaniyazova K, Azimzadeh S, Canıgür S, Esen F. Çevre Kirliliğinin İnsan Sağlığı Üzerindeki Etkileri [The effects of environmental pollution on human health]. Turk. J. Med. Res. [Internet]. 2019 [cited sept. 12 2024]; 1(3):52-58. Turkish. Available in: https://goo.su/zqAX
Güler Ç, Çobanoğlu Z. Pestisitler, çevre sağlığı [Pesticides, Environmental health essential]. Ankara (Türkiye): Ilkoz Printing Press; 1997. 169 p. Basic Resource Series, No: 52. Turkish.
Özay Ö, Arslantaş D. Pestisit maruziyeti ve nöropsikiyatrik etkileri [Pesticide exposure and neuropsychiatric effects]. Osmangazi Tıp Derg. [Internet]. 2016 [cited 10 Jul. 2024; 38(1):42-48. Available in: https://goo.su/B1L1yfV DOI: https://doi.org/10.20515/otd.88785
Goh PS, Ahmad NA, Wong TW, Yogarathinam LT, Ismail AF. Membrane technology for pesticide removal from aquatic environment: Status quo and way forward. Chemosphere 2022; 307(3):136018. doi: https://doi.org/gzhm4h DOI: https://doi.org/10.1016/j.chemosphere.2022.136018
Plant Protection Products Database. Ministry of Food, Agriculture and Livestock, Department of Plant Protection Products, Turkey. [Internet]. 2024 [cited 10 Jul. 2024]; Available in: https://goo.su/iFRP
Delen N, Durmuşoğlu E, Güncan A, Güngör N, Turgut C, Burçak A. Türkiye’de pestisit kullanımı, kalıntı ve organizmalarda duyarlılık azalışı sorunları [Problems of pesticide use, residues and reduced sensitivity of organisms in Turkey] [Internet]. Proceedings of 6th Technical Congress of Agricultural Engineering; 3-7 Jan. 2005 [cited 8 May 2024]; Ankara (Türkiye). 21 p. Turkish. Available in: https://goo.su/L3B0zY5
Sulak M, Çalhan R, Tulger G. Pestisitlerin taşınım süreçleri ve çevresel etkileri [Transport processes and environmental effects of pesticides]. Agricultural Health and Safety Symposium. 2012. p. 6-7. Turkish.
Altay O. İzmir körfezinde pestisit kirliliğinin araştırılması [The research of pesticide pollution in İzmir Bay], [Dissertation]. İzmir (Türkiye): Dokuz Eylül University; 1997. 37 p. Turkish.
Wurl O, Obbard JP. Chlorinated pesticides and PCBs in the sea–surface microlayer and seawater samples of Singapore. Mar. Pollut. Bull. [Internet]. 2005; 50(11):1233-1243. doi: https://doi.org/dw284j DOI: https://doi.org/10.1016/j.marpolbul.2005.04.022
Bobat A, Hengirmen OM, Zaplethal W. Tatlısu ekosisteminde teknik, ekonomik ve ekolojik bir zararlı: Zebra midye [A technical, economic and ecological pest in the freshwater ecosystem: Zebra mussel]. Ankara (Türkiye): Rural Environment and Forestry Problems Research Association; 2001. 127 p. Turkish. Rural Environment Yearbook.
Serdar O, Aydın R, Söylemez H. Effect of beta–cyfluthrin pesticide on Zebra mussel (Dressienna polymorpha). Int. J. Pure Appl. Sci. [Internet]. 2021; 7(3):462-471. doi: https://doi.org/g8zvht
Yıldırım F, Karasu Benli Ç, Gümüş BA, Erkmen B, Gül G, Batmaz G, Paçal E, Erkoç F. Biyosidal aktiviteli zinc pyrithione’un istilacı tür Dreissena polymorpha (Zebra midyesi)’ya akut toksisitesi [Acute toxicity of biocidal activity zinc pyrithione to the invasive species Dreissena polymorpha (Zebra Mussel)] Internet. Proceedings of 2nd National Biocidal Congress with International Participation; 3-9 Nov. 2015. Çeşme (Türkiye): Gazi University. Turkish.
Alişer AB. Zebra midyesi (Dreissena polymorpha)’nın kadmiyuma karşı bazı biyokimyasal yanıtları [Some biochemical responses against cadmium of Zebra mussels (Dressena polymorpha]. Master's thesis. Munzur (Türkiye), Munzur University; 2020 [cited 12 Apr. 2024]. 48 p. Turkish.
Aydın AN, Serdar O. Effect of probiotic on copper nanoparticle accumulation in Dreissena polymorpha. J. Limnol. Freshw. Fish. Res. [Internet]. 2024; 10(1):39-46. doi: https://doi.org/n9zv DOI: https://doi.org/10.17216/limnofish.1272399
Binelli A, Della Torre C, Magni, S, Parolini M. Does zebra mussel (Dreissena polymorpha) represent the freshwater counterpart of Mytilus in ecotoxicological studies? A critical review. Environ. Pollut. [Internet]. 2015; 196:386-403. doi: https://doi.org/f6w9gh DOI: https://doi.org/10.1016/j.envpol.2014.10.023
Serdar O. Determination of the effect of cyfluthrin pesticide on Zebra mussel (Dreissena polymorpha) by some antioxidant enzyme activities. JAES [Internet]. 2021; 6(1):77-83. doi: https://doi.org/gh3gcc DOI: https://doi.org/10.35229/jaes.804479
Erguven GÖ, Serdar O, Tanyol M, Yildirim NC, Yildirim N, Durmus B. The bioremediation capacity of Sphingomonas melonis for methomyl–contaminated soil media: RSM optimization and biochemical assessment by Dreissena polymorpha. ChemistrySelect [Internet]. 2022; 7(27):e202202105. doi: https://doi.org/n9zw DOI: https://doi.org/10.1002/slct.202202105
Serdar O, Aydin AN, Çimen ICÇ. Determination of oxidative stress responses caused by aluminum oxide (γ–Al2O3 and α–Al2O3) nanoparticles in Gammarus pulex. Chemosphere [Internet]. 2024; 352:141193. doi: https://doi.org/gvvfrz DOI: https://doi.org/10.1016/j.chemosphere.2024.141193
Kaur M, Jindal R. Oxidative stress response in liver, kidney and gills of Ctenopharyngodon idellus (cuvier & valenciennes) exposed to chlorpyrifos. MOJ Biol. Med. [Internet]. 2017; 1(4):103-112. doi: https://doi.org/n9zx DOI: https://doi.org/10.15406/mojbm.2017.01.00021
Subaramaniyam U, Allimuthu RS, Vappu S, Ramalingam D, Balan R, Paital B, Panda N, Rath PK, Ramalingam N, Sahoo DK. Effects of microplastics, pesticides and nano–materials on fish health, oxidative stress and antioxidant defense mechanism. Front. Physiol. [Internet]. 2023; 14:1217666. doi: https://doi.org/n9zz DOI: https://doi.org/10.3389/fphys.2023.1217666
Serdar O, Yildirim N, Yildirim NC. Single and combined effects of dimethoate and malathion on oxidative stress biomarkers in the non–target freshwater mussel Dreissena polymorpha. Oceanol. Hydrobiol. Stud. [Internet ]. 2023; 52(3):333-342. doi: https://doi.org/n9z2 DOI: https://doi.org/10.26881/oahs-2023.3.07
Modesto KA, Martinez CB. Roundup® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere [Internet]. 2010; 78(3):294-299. doi: https://doi.org/fswf63 DOI: https://doi.org/10.1016/j.chemosphere.2009.10.047
Frost J, Bennetsen MS. The use of biomarkers in ecotoxicology in the near future aquatic environment [Internet]. Roskilde (Denmark): Roskilde University, Enviromental Biology Project; 2019 [cited 16 Apr 2024]. 37 p. Available in: https://goo.su/TMM4P
Ji Y, Lu GH, Wang C, Zhang J. Biochemical responses of freshwater fish Carassius auratus to polycyclic aromatic hydrocarbons and pesticides. Water Sci. Eng. [Internet]. 2012; 5(2):145-154. doi: https://doi.org/n92q
Marins AT, Cerezer C, Leitemperger JW, Severo ES, Costa MD, Fontoura DO, Nunes MEM, Riveiro LC, Zanella R, Loro VL. A mixture of pesticides at environmental concentrations induces oxidative stress and cholinergic effects in the neotropical fish Rhamdia quelen. Ecotoxicol. [Internet]. 2021; 30:164-174. doi: https://doi.org/gv8c4w DOI: https://doi.org/10.1007/s10646-020-02300-6
Nwani CD, Lakra WS, Nagpure NS, Kumar R, Kushwaha B, Srivastava SK. Toxicity of the herbicide atrazine: effects on lipid peroxidation and activities of antioxidant enzymes in the freshwater fish Channa punctatus (Bloch). Int. J. Environ. Res. Public Health [Internet]. 2010; 7(8):3298-3312. doi: https://doi.org/dpfbr9 DOI: https://doi.org/10.3390/ijerph7083298
Sinhorin VDG, Sinhorin AP, dos Santos Teixeira JM, Miléski KML, Hansen PC, Moreira PSA, Kawashita NH, Baviera AM, Loro VL. Effects of the acute exposition to glyphosate–based herbicide on oxidative stress parameters and antioxidant responses in a hybrid Amazon fish surubim (Pseudoplatystoma sp). Ecotoxicol. Environ. Saf. [Internet]. 2014; 106:181-187. doi: https://doi.org/ggztht DOI: https://doi.org/10.1016/j.ecoenv.2014.04.040
Rossi AS, Fantón N, Michlig MP, Repetti MR, Cazenave J. Fish inhabiting rice fields: Bioaccumulation, oxidative stress and neurotoxic effects after pesticides application. Ecol. Indic. [Internet]. 2020; 113:106186. doi: https://doi.org/gwdz39 DOI: https://doi.org/10.1016/j.ecolind.2020.106186
Serdar O. The effect of dimethoate pesticide on some biochemical biomarkers in Gammarus pulex. Environ. Sci. Pollut. Res. [Internet]. 2019; 26:21905-21914. doi: https://doi.org/gwcp4w DOI: https://doi.org/10.1007/s11356-019-04629-w
Serdar O, Ketenalp Z, Cikcikoglu Yildirim N. The potential ameliorative impacts of rare earth element cerium in dimethoate induced toxicity in freshwater mussel Dreissena polymorpha. Chem. Ecol. [Internet]. 2023; 39(7):810-821. doi: https://doi.org/n92t DOI: https://doi.org/10.1080/02757540.2023.2256730
Söylemez H, Serdar O, Aydın R. Effect of beta–cyfluthrin pesticide on Zebra mussel (Dressienna polymorpha). Int. J. Pure Appl. Sci. [Internet]. 2021; 7(3):462-471. doi: https://doi.org/g8zvht DOI: https://doi.org/10.29132/ijpas.803520
Bhattacharya R, Chatterjee A, Chatterjee S, Saha NC. Acute toxicity and sublethal effects of sodium laureth sulfate on oxidative stress enzymes in benthic oligochaete worm, Tubifex tubifex. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. [Internet]. 2021; 243:108998. doi: https://doi.org/gwf66t DOI: https://doi.org/10.1016/j.cbpc.2021.108998
Yamano T, Morita S. Effects of pesticides on isolated rat hepatocytes, mitochondria, and microsomes II. Arch. Environ. Contam. Toxicol. [Internet]. 1995; 28:1-7. doi: https://doi.org/bb6src DOI: https://doi.org/10.1007/BF00213961
Ferreira D, Da Motta AC, Kreutz LC, Toni C, Loro VL, Barcellos LJG. Assessment of oxidative stress in Rhamdia quelen exposed to agrichemicals. Chemosphere [Internet]. 2010; 79(9):914-921. doi: https://doi.org/dqjghc DOI: https://doi.org/10.1016/j.chemosphere.2010.03.024
Cikcikoglu Yildirim N, Serdar O, Basaran S. The use of Gammarus pulex as a model organism for ecotoxicological assessment of ibuprofen and propranolol at environmental relevant concentrations. Int. J. Environ. Health Res. [Internet]. 2022; 32(11):2385-2395. doi: https://doi.org/g8rqgw DOI: https://doi.org/10.1080/09603123.2021.1967888
Livingstone DR. Contaminant–stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. [Internet]. 2001; 42(8):656- 666. doi: https://doi.org/dvg8st DOI: https://doi.org/10.1016/S0025-326X(01)00060-1
Lushchak VI, Lushchak LP, Mota AA, Hermes–Lima M. Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. Am. J. Physiol. Regul. Integr. Comp. Physiol. [Internet]. 2001; 280(1):R100-R107. doi: https://doi.org/n92w DOI: https://doi.org/10.1152/ajpregu.2001.280.1.R100
Marins AT, Severo E., Cerezer C, Leitemperger JW, Müller TE, Floriano L, Prestes OD, Zanella R, Loro VL. Environmentally relevant pesticides induce biochemical changes in Nile tilapia (Oreochromis niloticus). Ecotoxicol. [Internet]. 2021; 30:585-598. doi: https://doi.org/gn8n48 DOI: https://doi.org/10.1007/s10646-021-02368-8
Aydın AN, Serdar O, Parlak Ak T. Determination of toxic effect of gamma cyhalothrin in Dreissena polymorpha by some biomarkers. SSRN [Internet]. 2024; 4776051:1-27. Available in: https://doi.org/n922 DOI: https://doi.org/10.2139/ssrn.4776051
Moreno I, Pichardo S, Jos A, Gomez–Amores L, Mate A, Vazquez CM, Camean AM. Antioxidant enzyme activity and lipid peroxidation in liver and kidney of rats exposed to microcystin–LR administered intraperitoneally. Toxicon. [Internet]. 2005; 45(4):395-402. doi: https://doi.org/dszxbb DOI: https://doi.org/10.1016/j.toxicon.2004.11.001
Ballesteros M, Wunderlin DA, Bistoni MA. Oxidative stress responses in different organs of Jenynsia multidentata exposed to endosulfan. Ecotoxicol. Environ. Saf. [Internet]. 2009; 72(1):199-205. doi: https://doi.org/fs5trk DOI: https://doi.org/10.1016/j.ecoenv.2008.01.008
Yonar SM, Ural MŞ, Silici S, Yonar ME. Malathion–induced changes in the haematological profile, the immune response, and the oxidative/antioxidant status of Cyprinus carpio carpio: Protective role of propolis. Ecotoxicol. Environ. Saf. [Internet]. 2014; 102:202-209. doi: https://doi.org/f5vg4r DOI: https://doi.org/10.1016/j.ecoenv.2014.01.007