Relationship between racial predominance and ectoparasites in crossbreed cattle herds in a dry tropical weather. Technical note
Abstract
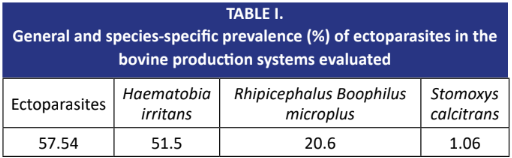
To estimate the ectoparasites prevalence and assess the impact of the racial predominance of Bos taurus taurus, Bos taurus indicus, and their crossbreeds on this prevalence in dual- purpose cattle herds in a tropical dry weather in Venezuela, a cluster sampling was conducted. 564 animals from twenty- two cattle production systems were sampled, proportionally distributed across six age groups (<3, 3-6, 6-12, 12-18, 18-32, and >32 months, respectively). The animals were evaluated for the presence of ectoparasites, with specimens collected for identification using dichotomous keys. Additionally, the variables of age and racial predominance were analyzed. The overall prevalence of ectoparasites was 57.54%, with specific prevalences of 51.5% for Haematobia irritans, 20.6% for Rhipicephalus (Boophilus) microplus, and 1.06% for Stomoxys calcitrans. There was a significant effect of age and racial predominance on the prevalence of ectoparasites overall, particularly for H. irritans and R. (B.) microplus, with a higher probability of infestation observed in adult animals and those predominantly B.t. taurus. The predominance of B.t. indicus and their crossbreeds was found to be a protective factor against ectoparasitosis. The relationship between ectoparasite prevalence and cattle racial predominance is clear, with B.t. indicus showing the highest resistance.
Downloads
References
Piper E, Jonsson N, Gondro C, Lew-Tabor A, Moolhuijzen P, Vance M, Jackson L. Immunological Profiles of Bos taurus and Bos indicus Cattle Infested with the Cattle Tick, Rhipicephalus (Boophilus) microplus. Clin. Vaccine Immunol. [Internet]. 2009; 16(7):1074-1086. doi: https://doi.org/dmwb9t DOI: https://doi.org/10.1128/CVI.00157-09
Franzin A, Maruyama S, Garcia G, Pereira R, Chaves J, Bishop R, Mendes A, Dantas D, Rossetti B, Ferreira I. Immune and biochemical responses in skin differ between bovine hosts genetically susceptible and resistant to the cattle tick Rhipicephalus microplus. Parasit. Vectors. [Internet]. 2017; 10:51. doi: https://doi.org/gntn8n DOI: https://doi.org/10.1186/s13071-016-1945-z
Basiel B, Hardie L, Heins B, Dechow C. Genetic parameters and genomic regions associated with horn fly resistance in organic Holstein cattle. J. Dairy Sci. [Internet]. 2021; 104(12):12724-12740. doi: https://doi.org/n93p DOI: https://doi.org/10.3168/jds.2021-20366
Shyma K, Prakash J, Singh V. Breeding strategies for tick resistance in tropical cattle: a sustainable approach for tick control. J. Parasit. Dis. [Internet]. 2015; 39:1-6. https://doi.org/n93q DOI: https://doi.org/10.1007/s12639-013-0294-5
Giachetto P, Casquero R, Nhani A, Garcia M, Apreciado J A, Andreotti R. Gene expression in the salivary gland of Rhipicephalus (Boophilus) microplus fed on tick-susceptible and tick-resistant hosts. Front. Cell. Infect. Microbiol. [Internet]. 2020; 9:477. doi: https://doi.org/n93r DOI: https://doi.org/10.3389/fcimb.2019.00477
Lugo M, Zambrano J, Fonseca C, Angulo F. Identificación de garrapatas (Acari: Ixodidae) en sistemas de producción bovina doble propósito del Cantón Chone, Provincia de Manabí, Ecuador. Nota Técnica. La Técnica [Internet]. 2023; 13(1):57-60. doi: https://doi.org/n93s DOI: https://doi.org/10.33936/latecnica.v13i1.5200
Salim S, Ahmed H, Sohail M, Riaz M, Birtles R, Oliver J. Epidemiology, Distribution and Identification of Ticks on Livestock in Pakistan. Int. J. Environ. Res. Public Health. [Internet]. 2022; 19(5):3024. doi: https://doi.org/n93t DOI: https://doi.org/10.3390/ijerph19053024
Chaiphongpachara, T, Duvallet G, Changbunjong T. Wing phenotypic variation among Stomoxys calcitrans (Diptera: Muscidae) populations in Thailand. Insects [Internet]. 2022; 13(5):405. doi: https://doi.org/n93v DOI: https://doi.org/10.3390/insects13050405
Andrade M, Giglioti R, Gutmanis G, Azevedo B, Fernandes C, Vercesi A, Katiki L, Veríssimo C. Selective control of Rhipicephalus microplus in a dairy cattle herd from different genetic groups. Braz. J. Vet. Parasitol. [Internet]. 2022; 31(4):e012622. doi: https://doi.org/n93w DOI: https://doi.org/10.1590/s1984-29612022062
Rehman A, Nijhof A, Sauter-Louis C, Schauer B, Staubach C, Conraths F. Distribution of ticks infesting ruminants and risk factors associated with high tick prevalence in livestock farms in the semiarid and arid agroecological zones of Pakistan. Parasit. Vectors [Internet]. 2017; 10:190. doi: https://doi.org/n93x DOI: https://doi.org/10.1186/s13071-017-2138-0
Ahmad N, Hashmi H. A comparative study on the incidence of ticks and ticks born diseases on local and cross- bred cattle in Malakand agency. J. Anim. Plant. Sci. [Internet]. 2007; 17(3-4):56-58. Disponible en: https://goo.su/ luDNH
Alves M, Medeiros A, Riet F, Marques A, Gomes JG, Vieira VD, Miraballes C. Identification of Sindhi cows that are susceptible or resistant to Haematobia irritans. Braz. J. Vet. Parasitol. [Internet]. 2019; 28(3), 465-472. doi: https://doi.org/n93z DOI: https://doi.org/10.1590/s1984-29612019066
Fuenmayor W. Síntesis socio histórico, cultural y geográfico. Atlas estado Zulia. 5ª Ed. Editorial Splanos C.A. 2005; p 110-113.
Casal J, Mateu E. Tipos de muestreo. Rev. Epidem. Med.Prev. [Internet]. 2003; [cited August 09 2024]; 1:3-7. Available in: https://goo.su/gztN2yE
Wall R, Shearer D. Veterinary ectoparasites: Biology, pathology and control. Second Edition. Oxford, England: Blackwell Science Ltd. 2001. DOI: https://doi.org/10.1002/9780470690505
Margolis L, Esch GW, Holmes JC, Kuris AM, Schad GA. The use of ecological terms in parasitology. (Report of an Ad Hoc Committee of the American Society of Parasitologists). J. Parasitol. [Internet]. 1982; [cited September 15 2024]; 68(1):131-133. Available in: https://goo.su/hIvyd DOI: https://doi.org/10.2307/3281335
Jian W, Duangjinda M, Vajrabukka C, Katawatin S. Differences of skin morphology in Bos indicus, Bos taurus, and their crossbreds. Int. J. Biometeorol. [Internet]. 2014; 58:1087–1094. doi: https://doi.org/f6bj6p DOI: https://doi.org/10.1007/s00484-013-0700-9
Mohamed A, Fedlu M, Nigussie T, Wali M A. Prevalence, seasonal dynamics and associated variables of ixodid tick cattle infestation in Gondar, northwestern Ethiopia. Parasit. Epidemiol. Control. [Internet]. 2023; 21:e00294. doi: https://doi.org/n932 DOI: https://doi.org/10.1016/j.parepi.2023.e00294
Araújo WA, Ianella P, Arnoldi F, Caetano A, Maruyama S, Ferreira BR, Conti LH, Da Silva MR, Paula JF, Maia AA, De Miranda IKF. Haplotypes of the bovine IgG2 heavy gamma chain in tick-resistant and tick-susceptible breeds of cattle. Immunogenetics. [Internet]. 2011; 63:319-324. doi: https://doi.org/b4b6cb DOI: https://doi.org/10.1007/s00251-011-0515-y
Tabor AE, Ali A, Rehman G, Rocha Garcia G, Zangirolamo AF, Malardo T, Jonsson NN. Cattle tick Rhipicephalus microplus-host interface: A review of resistant and susceptible host responses. Front. Cell. Infect. Microbiol. [Internet]. 2017; 7:506. doi: https://doi.org/gcstdd DOI: https://doi.org/10.3389/fcimb.2017.00506
Rocha JF, Martínez R, López-Villalobos N, Morris ST. Tick burden in Bos taurus cattle and its relationship with heat stress in three agroecological zones in the tropics of Colombia. Parasit. Vectors [Internet]. 2019; 12:73. https://doi.org/n933 DOI: https://doi.org/10.1186/s13071-019-3319-9
Raza A, Schulz BL, Nouwens A, Naseem MN, Kamran M, Mantilla Valdivieso EF, Kerr ED, Constantinoiu C, Jonsson NN, James P, Tabor AE. Application of quantitative proteomics to discover biomarkers for tick resistance in cattle. Front. Immunol. [Internet]. 2023; 14:1091066. doi: https://doi.org/g5k55x DOI: https://doi.org/10.3389/fimmu.2023.1091066
Barros JC, Garcia MV, de Oliveira SHL, da Silva SA, Andreotti A. Profile of cattle breed sensitivity to the tick Rhipicephalus microplus. Ticks and Tick-borne Dis. [Internet]. 2024; 15(5):102363. doi: https://doi.org/n934 DOI: https://doi.org/10.1016/j.ttbdis.2024.102363
Mendes J, Linhares A. Cattle Dung Breeding Diptera in pastures in southeastern Brazil: Diversity, abundance and seasonallity. Mem. Inst. Oswaldo Cruz, Rio de Janeiro. [Internet]. 2002; 97(1):37-41. doi: https://doi.org/bwsxgc DOI: https://doi.org/10.1590/S0074-02762002000100004