The effect of acute Carbon Monoxide intoxication on cardiac necrosis in rats: in relation to Adiponectin levels
Abstract
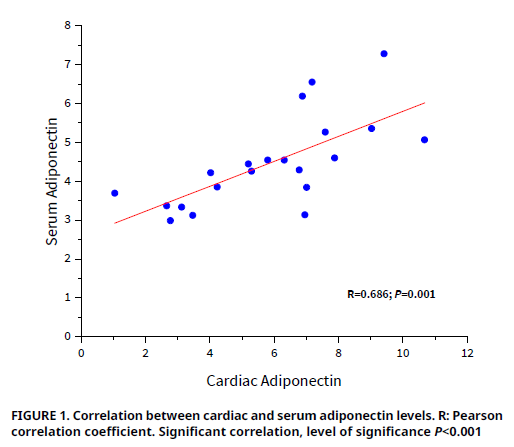
In order to investigate the effects of acute CO poisoning and subsequent oxygen therapy on cardiac necrosis in rats, with a specific focus on adiponectin levels, twenty–one male Wistar albino rats were divided into three groups (Control, CO, CO+O2). The Control group was placed in a container and exposed to room air for 30 min. Acute CO poisoning was induced in the CO group and CO+O2 group by exposing the rats to CO gas for 30 min. Following CO exposure, the CO+O2 group received oxygen therapy for 30 min, while the CO group did not receive any additional intervention. The animals were euthanized by cardiac puncture under anesthesia, following the approved ethical procedures. Carboxyhemoglobin (COHb), serum levels of creatine kinase (CK), creatine kinase myocardial band (CK–MB), C–reactive protein (CRP) and lactate dehydrogenase (LDH), as well as cardiac and serum adiponectin levels were measured. CO poisoning caused necrosis in cardiac tissue however, oxygen therapy alleviated the negative effect of CO on cardiac injury. COHb and LDH levels in CO group were increased, whereas both cardiac and serum adiponectin levels were decreased (all, P<0.05). There were no changes in CK, CK–MB, CRP levels among groups (all, P>0.05). Oxygen therapy decreased COHb, but increased both cardiac and serum adiponectin levels (all, P<0.05). Adiponectin and LDH may serve as potential biomarkers for early diagnosis of cardiac necrosis caused by acute CO poisoning. The assessment or quantification of adiponectin can also be useful for the early prognosis of cardiac necrosis after oxygen therapy.
Downloads
References
Yuan Z, De La Cruz LK, Yang X, Wang B. Carbon monoxide signaling: examining ıts engagement with various molecular targets in the context of binding affinity, concentration, and biologic response. Pharmacol Rev. [Internet]. 2022; 74(3):825-875 . doi: https://doi.org/n7rs DOI: https://doi.org/10.1124/pharmrev.121.000564
Adach W, Błaszczyk M, Olas B. Carbon monoxide and its donors – Chemical and biological properties. Chem. Biol. Interact. [Internet]. 2020; 318:108973. doi: https://doi.org/gpkqrb DOI: https://doi.org/10.1016/j.cbi.2020.108973
Sobhakumari A, Poppenga RH, Pesavento JB, Uzal FA. Pathology of carbon monoxide poisoning in two cats. BMC Vet. Res. [Internet]. 2018; 14:67. doi: https://doi.org/n7rz DOI: https://doi.org/10.1186/s12917-018-1385-4
Ito H, Ogawa R, Shimojo N. Rhabdomyolysis secondary to carbon monoxide poisoning: A retrospective cohort study. Am. J. Emerg. Med. [Internet]. 2022; 60:207-208. doi: https://doi.org/n7r4 DOI: https://doi.org/10.1016/j.ajem.2022.06.051
Geng S, Hao X, Xu H, Yao J, He D, Xin H, Gong X, Zhang R.Cardiac injury after acute carbon monoxide poisoning and its clinical treatment scheme. Exp. Ther. Med. [Internet]. 2020; 20(2):1098-1104. doi: https://doi.org/n7r5 DOI: https://doi.org/10.3892/etm.2020.8801
Koga H, Tashiro H, Mukasa K, Inoue T, Okamoto A, Urabe S, Sagara S, Yano K, Onitsuka K, Yamashita H. Can indicators of myocardial damage predict carbon monoxide poisoning outcomes? BMC Emerg. Med. [Internet]. 2021; 21(1):7. doi: https://doi.org/n7r6 DOI: https://doi.org/10.1186/s12873-021-00405-7
Alva R, Mirza M, Baiton A, Lazuran L, Samokysh L, Bobinski A, Cowan C, Jaimon A, Obioru D, Al Makhoul T, Stuart JA. Oxygen toxicity: cellular mechanisms in normobaric hyperoxia. Cell. Biol. Toxicol. [Internet]. 2023; 39(1):111-143. doi: https://doi.org/g7xrvd DOI: https://doi.org/10.1007/s10565-022-09773-7
Maghamiour N, Safaie N. High creatine kinase (CK)–MB and lactate dehydrogenase in the absence of myocardial injury or infarction: A case report. J. Cardiovasc. Thorac. Res. [Internet]. 2014; 6:69-70. doi: https://doi.org/n7r7
Bojinca M, Bojinca VC, Balanescu AR, Balanescu SM. Macro creatine kinase (macro CK) in clinical practice. Rev. Chim. [Internet]. 2018; 69(8):2107-2109. doi: https://doi.org/n7r8 DOI: https://doi.org/10.37358/RC.18.8.6483
Farhana A, Lappin SL. Biochemistry, Lactate Dehydrogenase. [Internet]. Treasure Island (FL, USA): StatPearls Publishing; 2024 [cited 05 May 2024]. Available in: https://goo.su/hoybTL
Peng J, Chen Q, Wu C. The role of adiponectin in cardiovascular disease. Cardiovasc. Pathol. [Internet]. 2023; 64:107514. doi: https://doi.org/g7v978 DOI: https://doi.org/10.1016/j.carpath.2022.107514
Karastergiou K, Evans I, Ogston N, Miheisi N, Nair D, Kaski JC, Jahangiri M, Mohamed–Ali V. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscl. Throm. Vas. [Internet]. 2010; 30(7):1340-1346. doi: https://doi.org/dsrtzh DOI: https://doi.org/10.1161/ATVBAHA.110.204719
Guo J, Zhu K, Li Z, Xiao C. Adiponectin protects hypoxia/Reoxygenation–induced cardiomyocyte injury by suppressing autophagy. J. Immunol. Res. [Internet]. 2022; 2022:e8433464. doi: https://doi.org/n7r9 DOI: https://doi.org/10.1155/2022/8433464
Khan RS, Kato TS, Chokshi A, Chew M, Yu S, Wu C, Singh P, Cheema FH, Takayama H, Harris C, Reyes–Soffer G, Knöll R, Milting H, Naka Y, Mancini D, Schulze PC. Adipose tissue inflammation and adiponectin resistance in patients with advanced heart failure: correction after ventricular assist device implantation. Circ. Heart Fail. [Internet]. 2012; 5(3):340-348. doi: https://doi.org/f3vc3m DOI: https://doi.org/10.1161/CIRCHEARTFAILURE.111.964031
Gandini C, Castoldi AF, Candura SM, Priori S, Locatelli C, Butera R, Bellet C, Manzo L. Cardiac damage in pediatric carbon monoxide poisoning. J. Toxicol. Clin. Toxicol. [Internet]. 2001; 39(1):45-51. doi: https://doi.org/dmxrmp DOI: https://doi.org/10.1081/CLT-100102879
Gokdemir GS, Seker U, Demirtas B, Taskin S. Effects of acute carbon monoxide poisoning on liver damage and comparisons of related oxygen therapies in a rat model. Toxicol. Mech. Method. [Internet]. 2024; 34(8):845-854. doi: https://doi.org/n7sb DOI: https://doi.org/10.1080/15376516.2024.2353887
Brvar M, Mozina M, Osredkar J, Suput D, Bunc M. Prognostic value of S100B protein in carbon monoxide–poisoned rats. Crit. Care Med. [Internet]. 2004; 32(10):2128-2130. doi: https://doi.org/bb66km DOI: https://doi.org/10.1097/01.CCM.0000142702.39718.A0
Bishop ML, Fody, EP, Schoeff LE. Clinical Chemistry: principles, techniques, and correlations. 5th ed. Baltimore (MD, USA): Lippincott Williams & Wilkins; 2005. 756 p.
Ortiz–Avila O, García–Berumen CI, Figueroa–García MdC, Mejía–Zepeda R, Saavedra–Molina A, Meléndez–Herrera E, Cortés–Rojo C. Avocado oil delays kidney ınjury by ımproving serum adiponectin levels and renal mitochondrial dysfunction in type 2 diabetic rats. J. Biol. Regul. Homeost. Agents. [Internet]. 2024; 38(3):1975–1985. doi: https://doi.org/n7sc DOI: https://doi.org/10.23812/j.biol.regul.homeost.agents.20243803.154
Tabrizian K, Shahriari Z, Rezaee R, Jahantigh H, Bagheri G, Tsarouhas K, Docea AO, Tsatsakis A, Hashemzaei M. Cardioprotective effects of insulin on carbon monoxide–induced toxicity in male rats. Hum. Exp. Toxicol. [Internet]. 2019; 38(1):148-154. doi: https://doi.org/n7sd DOI: https://doi.org/10.1177/0960327118788134
Singh P. P value, statistical significance and clinical significance. J Clin Prev Cardiol. [Internet]. 2013[cited August 15 2024]; 2(4):202-204. Available in: https://goo.su/YrYoB0
Orhan Ö, Yeşil A. Carbon monoxide poisoning: comparison of paediatrics and adult patients. Eurasian J. Tox. [Internet]. 2023; 5(2):28-31 doi: https://doi.org/n7sf DOI: https://doi.org/10.51262/ejtox.1313935
Veiraiah A. Carbon monoxide poisoning. Medicine [Internet]. 2020; 48(3):197-198. doi: https://doi.org/n7sg DOI: https://doi.org/10.1016/j.mpmed.2019.12.013
Lee KK, Spath N, Miller MR, Mills NL, Shah ASV. Short–term exposure to carbon monoxide and myocardial infarction: A systematic review and meta–analysis. Environ. Int. [Internet]. 2020; 143:105901. doi: https://doi.org/gwbtrn DOI: https://doi.org/10.1016/j.envint.2020.105901
Dent MR, Rose JJ, Tejero J, Gladwin MT. Carbon monoxide poisoning: from microbes to therapeutics. Annu. Rev. Med. [Internet]. 2024; 75:337-351. doi: https://doi.org/n7sh DOI: https://doi.org/10.1146/annurev-med-052422-020045
Haliga RE, Morărașu BC, Șorodoc V, Lionte C, Sîrbu O, Stoica A, Ceasovschih A, Constantin M, Șorodoc L. Rare causes of acute coronary syndrome: carbon monoxide poisoning. Life [Internet]. 2022; 12(8):1158. doi: https://doi.org/n7sj DOI: https://doi.org/10.3390/life12081158
Savioli G, Gri N, Ceresa IF, Piccioni A, Zanza C, Longhitano Y, Ricevuti G, Daccò M, Esposito C, Candura SM. Carbon monoxide poisoning: from occupational health to emergency medicine. J. Clin. Med. [Internet]. 2024; 13(9):2466. doi: https://doi.org/g8wmpj DOI: https://doi.org/10.3390/jcm13092466
Abo El–Noor M, Elgazzar FM, El–Shafy G, Shouip OM. Serum acute phase proteins as novel markers of myocardial ınjury in acute carbon monoxide poisoned patients. Mansoura J. Forensic Med. Clin. Toxicol. [Internet]. 2016; 24(2):17-33. doi: https://doi.org/n7sk DOI: https://doi.org/10.21608/mjfmct.2016.47802
İpek S, Güllü UU, Güngör Ş, Demiray Ş. The effect of full blood count and cardiac biomarkers on prognosis in carbon monoxide poisoning in children. Ir. J. Med. Sci. [Internet]. 2023; 192(5):2457-2466. doi: https://doi.org/n7sm DOI: https://doi.org/10.1007/s11845-022-03232-2
Lewandrowski K, Chen A, Januzzi J. Cardiac markers for myocardial infarction. A brief review. Am. J. Clin. Pathol. [Internet]. 2002; 118(Suppl 1): 93-99. doi: https://doi.org/bwzqbr DOI: https://doi.org/10.1309/3EK7-YVV9-228C-E1XT
Akcali G, Uzun G, Arziman I, Aydin I, Yildiz S. The relationship between intoxication severity and blood interleukin 6, interleukin 10 and CRP levels in carbon monoxide–poisoned patients. Undersea Hyperb. Med. 2018; 45(6):646–652. PMID: 31158931. DOI: https://doi.org/10.22462/11.12.2018.4
Kim YO, Kim HI, Jung BK. Pattern of change of C–reactive protein levels and its clinical implication in patients with acute poisoning. SAGE Open Med. [Internet]. 2022; 10:2022. doi: https://doi.org/g6dzt3 DOI: https://doi.org/10.1177/20503121211073227
Hernández Bello CY, Figueroa–UribeAF, Hernández–Ramírez J. Biochemical suffocants: Carbon monoxide and Cyanide. Rev. Fac. Med. Hum. [Internet]. 2022; 22(3):614-624. doi: https://doi.org/n7sn DOI: https://doi.org/10.25176/RFMH.v22i3.4928
Agoro ES, Chinyere GC, Akubugwo EI, Wankasi MM, Agi VN. Some vitreous humour cardiorenal biochemical parameters as an indicator of acute carbon monoxide poisoning death: an animal model. Australian. J. Forens. Sci. [Internet]. 2019; 51(4):476-484. doi: https://doi.org/n7sp DOI: https://doi.org/10.1080/00450618.2018.1429015
Khalaf M, El–Desouky N, El–Galad G, Abbas AH. Acute carbon monoxide–induced cardiotoxicity: clinical study. Int. J. Med. Toxicol. Leg. Med. [Internet]. 2011 [cited 18 September 2024]; 14:28-36. Available in: https://goo.su/lqsrtq
Feijóo–Bandín S, Aragón–Herrera A, Moraña–Fernández S, Anido–Varela L, Tarazón E, Roselló–Lletí E, Portolés M, Moscoso I, Gualillo O, González–Juanatey JR, Lago F. Adipokines and inflammation: focus on cardiovascular diseases. Int. J. Mol. Sci. [Internet]. 2020; 21(20):7711. doi: https://doi.org/gpxqsf DOI: https://doi.org/10.3390/ijms21207711
Nielsen MB, Çolak Y, Benn M, Mason A, Burgess S, Nordestgaard BG. Plasma adiponectin levels and risk of heart failure, atrial fibrillation, aortic valve stenosis, and myocardial infarction: large–scale observational and Mendelian randomization evidence. Cardiovasc. Res. [Internet]. 2024; 120(1):95-107. doi: https://doi.org/g8v9sg DOI: https://doi.org/10.1093/cvr/cvad162
Fu L, Du J, Furkert D, Shipton ML, Liu X, Aguirre T, Chin AC, Riley AM, Potter BVL, Fiedler D, Zhang X, Zhu Y, Fu C. Depleting inositol pyrophosphate 5-InsP7 protected the heart against ischaemia–reperfusion injury by elevating plasma adiponectin. Cardiovasc. Res. [Internet]. 2024; 120(8):954-970. doi: https://doi.org/n7sq DOI: https://doi.org/10.1093/cvr/cvae017
Varma D, Mulay S,Chemtob S. Carbonmonoxide: from public health risk to painless killer. In: Gupta RC, editor. Handbook of toxicology of chemical warfare agents [Internet]. Amsterdam (Netherlands): Academic Press; 2009. p. 271-292. doi: https://doi.org/fhxbf8 DOI: https://doi.org/10.1016/B978-0-12-374484-5.00020-1
Ashbaugh EA, Mazzaferro EM, McKiernan BC, Drobatz KJ. The association of physical examination abnormalities and carboxyhemoglobin concentrations in 21 dogs trapped in a kennel fire. J. Vet. Emerg. Crit. Care. [Internet]. 2012; 22(3):361-367. doi: https://doi.org/f35wdb DOI: https://doi.org/10.1111/j.1476-4431.2012.00759.x
Huang CC, Chen TH, Ho CH, Chen YC, Hsu CC, Lin HJ, Wang JJ, Chang CP, Guo HR. Increased risk of congestive heart failure following carbon monoxide poisoning. Circ. Heart Fail. [Internet]. 2021; 14(4):478-487. doi: https://doi.org/gq4ss3 DOI: https://doi.org/10.1161/CIRCHEARTFAILURE.120.007267
Cardiga R, Proença M, Carvalho C, Costa L, Botella A, Marques F, Paulino C, Carvalho A, Fonseca C. Intoxicação por monóxido de carbono com compromisso cardíaco: o que sabemos? Rev. Port. Cardiol. [Internet]. 2015; 34(9):557.e1-557.e5. Portuguese. doi: https://doi.org/f3hbqw DOI: https://doi.org/10.1016/j.repc.2015.01.006
Copyright (c) 2025 Gul Sahika Gökdemir, Sümeyye Çakmak, Berjan Demirtas, Mehmet Tahir Gökdemir, Ozgur Sogut, Revşa Evin Canpolat–Erkan, Fırat Aşır, Beran Yokus

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.


















