The effect of Cellulases and Xylanases on the in vitro digestibility of Asparagus browse (Asparagus officinalis), Corn stover (Zea mays) and Peanut hulls (Arachis hypogaea) in ruminants
Abstract
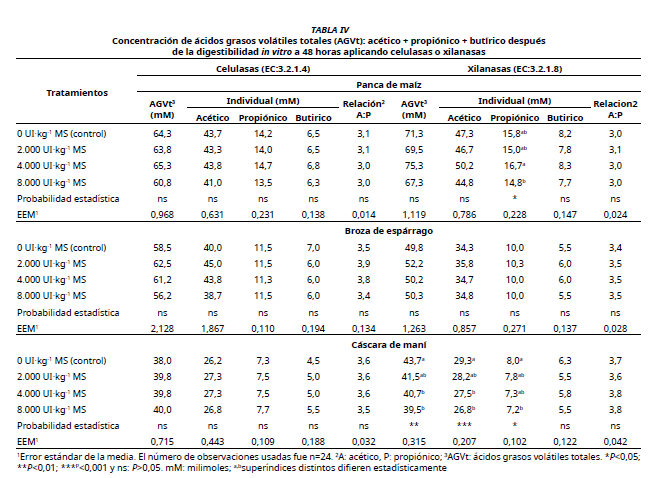
Crop residues play an important role in animal production worldwide. Improving the nutritional potential of low-quality options with fibrolytic enzymes would improve forage digestibility and utilization. Using an in vitro method, it was evaluated the effect of Cellulases (EC: 3.2.1.4) and Xylanases (EC: 3.2.1.8) applied at 4 levels: 0 (control), 2,000; 4,000 and 8,000 UI·kg-1 MS in Corn stover (CS), Asparagus browse (AB) and Peanut hulls (PH). When applying Cellulases to CS, the in vitro digestibility of dry matter (IVDMD) and the in vitro digestibility of neutral detergent fiber (IVNDFD) were higher (P<0.001) than the control group (63.7 vs. for 61.8% and 51.9 vs. 50.1%). Similar effects were found with Xylanases (64.1 vs. for 61.8% and 53.0 vs. 51.6%). The IVDMD and IVNDFD of AB were not affected by the application of Cellulases or Xylanases. In the case of PH, the application of Cellulases or Xylanases improved the IVDMD (24.9 vs. 22.3% and 24.6 vs. 22.3%), but not the IVNDFD. Also, the in vitro gas production at 48 hours was not influenced by the type of enzymes or by their levels of application to the residues evaluated. Cellulases or Xylanases applied to CS and AB had no effect on the concentration of tVFA (acetic acid + propionic acid + butyric acid). In the case of PH, the concentration of tVFA was similar between the control and those treated with Cellulases, while the application of Xylanases resulted in lower concentrations of tVFA than the control. Cellulases and Xylanases influenced IVDMD, IVNDFD and the concentration of tVFA depending on the substrate used.
Downloads
References
ADESOGAN, A; MA, Z; ROMERO, J; ARRIOLA, K. Ruminant Nutrition Symposium: Improving cell wall digestion and animal performance with fibrolytic enzymes. J. Anim. Sci. 92(4): 1317–1330. 2014.
ASSOCIATION OF OFFICIAL AGRICULTURE CHEMIST (AOAC). Molecular biology methods. Official Methods of Analysis. Washington, D.C. 125 pp. 2005.
ARANDA, E; MENDOZA, G; RAMOS, J; DA SILVA, I; VITTI, A. Effect of fibrolytic enzymes on rumen microbial degradation of sugarcane fiber. J. Sci. Anim. Brasilian. Goiania. 11(3): 488–495. 2010.
ARRIOLA, K; KIM, S; STAPLES, C; ADESOGAN, A. Effect of fibrolytic enzyme application to low-and high-concentrate diets on the performance of lactating dairy cattle. J. Dairy Sci. 94: 832–841. 2011.
AVELLANEDA, J; GONZALES, S; PINOS-RODRÍGUEZ, J; HERNÁNDEZ, A; MONTANEZ, O; SEGUERA, J. Enzimas fibrolíticas exógenas en la digestibilidad in vitro de cinco ecotipos de Brachiaria. Agron. Mesoamer. 18(1): 11–17. 2007.
BAILEY, M; BIELY, P; POUTANEN, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotech. 23: 257–270. 1992.
BEAUCHEMIN, K; RODE, L; MAEKAWA, M; MORGAVI, D; KAMPEN, R. Evaluation of a nonstarch polysaccharidase feed enzyme in dairy cow diets. J. Dairy Sc. 83: 543–553. 2003.
BEAUCHEMIN, K; HOLTSHAUSEN, L. Developments in Enzyme Usage in Ruminants. Enzymes in Farm Animal Nutrition. 2nd. Ed. CABI Pub. Cambridge. Pp 206–225. 2010.
CARREÓN, L; PINOS-RODRÍGUEZ, J; BÁRCENA, R; GONZALES, S; MENDOZA, G. Influence of fibrolytic enzymes on ruminal disappearance and fermentation in steers fed diets with short and long particle length of forage. Italian J. Anim. Sci. 9 (e17): 83–87. 2010.
COLOMBATTO, D; HERVÁS, G; YANG, W; BEAUCHEMIN, K. Effects of enzyme supplementation of a total mixed ration on microbial fermentation in continuous culture, maintained at high and low pH. J. Anim. Sci. 81: 2617–2627. 2003.
DEAN, D; ADESOGAN, A; KRUEGER, N; LITTELL, R. Effect of fibrolytic enzymes on the fermentation characteristics, aerobic stability, and digestibility of Bermuda grass silage. J. Dairy Sci. 88: 994–1003. 2005.
ELWAKEEL, E; TITGEMEYER, E; JOHNSON, B; ARMENDARIZ, C; SHIRLEY, J. Fibrolytic enzymes to increase the nutritive value of dairy feed stuffs. J. Dairy Sci. 90: 5226–5236. 2007.
ERWIN, E; MARCO, G; EMERY, E. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44: 1768–1771. 1961.
EUN, J; BEAUCHEMIN, K. Enhancing in vitro degradation of Alfalfa hay and corn silage using feed enzymes. J. Dairy Sci. 90: 2839–2851. 2007.
EUN, J; BEAUCHEMIN, K; SCHULZE, H. Use of an in vitro fermentation biosssay to evaluate improvements in degradation of alfalfa hay due to exogenous feed enzymes. J. Anim. Feed Sci. 135: 315–328. 2007a.
EUN, J; BEAUCHEMIN, K; SCHULZE, H. Use of exogenous fibrolytic enzymes to enhance in vitro fermentation of Alfalfa hay and corn silage. J. Dairy Sci. 90: 1440–1451. 2007b.
FLACHOWSKY, G. Carbon-footprints for food of animal origin, reduction potentials and research need. J. Appl. Anim. Res. 39(1): 2–14. 2011.
GIRALDO, L; TEJIDO, M; RANILLAAND, M; CARRO, M. Effects on exogenous fibrolytic enzymes on in vitroruminal fermentation of substrates ratios. J. Anim. Feed Technol. 141: 306–325. 2008a.
GIRALDO, L; TEJIDO, M; RANILLAAND, M; CARRO, M. Influence of direct-fed fibrolytic enzymes on diet digestibility and ruminal activity in sheep fed a grass hay-based diet. J. Anim. Sci. 86: 1617–1623. 2008b.
GOERING, H; VAN SOEST, P. Forage fiber analisis (apparatus, reagents, procedures and some applications). Agric. Handbook. Nº 379. ARS–USDA. Washington, DC, USA. 20 pp. 1970.
GÓMEZ, A; MENDOZA, G; PINOS, J. Comparison of in vitro degradation of elephant grass and sugarcane by exogenous fibrolytic enzymes. African J. Microbiol. Res. 5 (19): 3051–3053. 2011.
HOLTSHAUSEN, L; CHUNG, Y; GERARDO, H; OBA, M; BEAUCHEMIN, K. Improved milk production efficiency in early lactation dairy cattle with dietary addition of a developmental fibrolytic enzyme additive. J. Dairy Sci. 94: 899–907. 2011.
JALILVAND, G; ODONGO, N; LÓPEZ, S; NASERIA, A; VALIZADEH, R; EFTEKHAR, F; KEBREAB, E; FRANCE, J. Effects of different level of an enzyme mixture on in vitro gas production parameter of contrasting forage. J. Anim. Feed Sci. Technol. 146: 289–301. 2008.
KHATTAB, M; TAWAB, A. In vitro evaluation of palm fronds as feedstuff on ruminal digestibility and gas production. Acta Scientif. Anim. Sci. 40: e39586. 2018.
MALIK, R; BANDLA, S. Effect of source and dose of probiotics and exogenous fibrolytic enzymes (EFE) on intake, feed efficiency and growth of male buffalo (Bubalus bubalis) calves. Tropic. Anim. Health Prod. 42(6): 1263–1269. 2010.
MAURICIO, R; MOULD, F; DHANOA, M; OWEN, E; CHANNA, K; THEODOROU, M. Asemi-automated in vitro gas production technique for ruminant feedstuff evaluation. Feed Sci. Technol. 79(4): 321–330.1999.
MCALLISTER, T; HRISTOV, A; BEAUCHEMIN, K; RODE, L: CHENG, K. Enzymes in ruminants diets. In: Bedford, M.R.; Partridge, G.G. (Eds.). Enzymes in Farm Animal Nutrition. CAB International, Wiltshire. Pp 273–298. 2001.
MEALE, S; BEAUCHEMIN, K; HRISTOV, A; CHAVES, A; MCALLISTER, T. Board-Invited review: Opportunities and challenges in using exogenous enzymes to improve ruminant production. J. Anim. Sci. 92: 427–442. 2013.
MEDINA, M; TIRADO, G; MEJÍA, I; CAMARILLO, I; CRUZ, C. Digestibilidad in situ de dietas con harina de nopal deshidratado conteniendo un preparado de enzimas fibrolíticas exógenas. Pesquisa Agrop. Brasil. 41(7): 1173–1177. 2006.
MENDOZA, G; LOERA, O; PLATA, F; HERNÁNDEZ, P; RAMÍREZ, M. Considerations on the use of exogenous fibrolytic enzymes to improve forage utilization. The Scientif. World J. 2014: e247437. 2014.
MORENO, R; PINOS, J; GONZÁLES, S; ÁLVAREZ, G; GARCÍA, J; MENDOZA, G; BÁRCENA, R. Efecto de enzimas fibrolíticas exógenas en la degradación ruminal in vitro de dietas para vacas lecheras. J. Inter. 32(12) 850–853. 2007.
RAN, T; SALEEM, A; SHEN, Y; RIBEIRO, G; BEAUCHEMIN, K; TSANG, A; MCALLISTER, T. Effects of a recombinant fibrolytic enzyme on fiber digestion, ruminal fermentation, nitrogen balance, and total tract digestibility of heifers fed a high forage diet. J. Anim. Sci. 97(8): 3578–3587. 2019.
RIBEIRO, G; BADHAN, A; HUANG, J; BEAUCHEMIN, K; YANG, Z; WANG, Y; TSANG, A; MCALLISTER, T. New recombinant fibrolytic enzymes for improved in vitro ruminal fiber degradability of barley straw. J. Anim. Sci. 96: 3928–3942. 2018. https://doi.org/jb4w.
ROMERO, J; ZARATE, M; QUEIROZ, O; HAN, J; SHIN, J; STAPLES, C; BROWN, W; ADESOGAN, A. Fibrolytic enzyme and ammonia application effects on the nutritive value, intake, and digestion kinetics of bermuda grass hay in beef cattle. J. Anim. Sci. 91: 4345–4356. 2013.
ROMERO, J; ZARATE, M; ADESOGAN, A. Effect of the dose of exogenous fibrolytic enzyme preparation on preingestive fiber hydrolysis, ruminal fermentation, and in vitro digestibility of Bermuda grass haylage. J. Dairy Sci. 98: 406–417. 2015.
STATISTICAL ANALYSIS SYSTEM INSTITUTE (SAS). The SAS System for Microsoft Windows, release 8.2. SAS. 2001.
SELZER, K; HASSEN, A; AKANMU, A; SALEM, A. Digestibility and rumen fermentation of a high forage diet pre-treated with a mixture of cellulase and xylanase enzymes. South Afric. J. Anim. Sci. 51(3): 399–406. 2021.
SUTTON, J; PHIPPS, R; BEEVER, D; HUMPHRIES, D; HARTNELL, G; VICINI, J; HARD, D. Effect of method of application of a fibrolytic enzyme product on digestive processes and milk production in Holstein-Friesian cows. J. Dairy Sci. 86: 546–556. 2003.
TANG, S; TAYO, G; TAN, Z; SUN, Z; SHEN, L; SHOW, C; XIAO, C; REN, G; HAN, X; SHEN, S. Effects of yeast culture and fibrolytic enzyme supplementation on in vitro fermentation characteristics of low-quality cereal straws. J. Anim Sci. 86: 1164–1172. 2008.
TITI, H; TABBAA, M. Efficacy of exogenous cellulase on digestibility in lambs and growth of dairy calve. J. Livest. Prod. Sci. J. 87: 207–214. 2004.
VAN SOEST, P; ROBERTSON, J; LEWIS, B. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74: 3583–3597. 1991.
WANG, Y; SPRATLING, B; ZOBELL, D; WIEDMEIER, R; MCALLISTER, T. Effect of alkali pretreatment of wheat straw on the efficacy of exogenous fibrolytic enzymes. J. Anim. Sci. 82: 198–208. 2004.
WOOD, T; BHAT, K. Methods for measuring cellulase activities. En: Methods in Enzymology. Academic Press. Pp 87–112. 1988.
Copyright (c) 2022 Edis Geovanny Macías-Rodríguez, Carlos Alfredo Gómez-Bravo, Jimmy Roberto Álava-Moreira, Ernesto Antonio Hurtado

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.


















