Efectos antioxidantes y antiinflamatorios del extracto de Helichrysum plicatum DC. subsp. plicatum, en un modelo experimental de urolitiasis aguda
Resumen
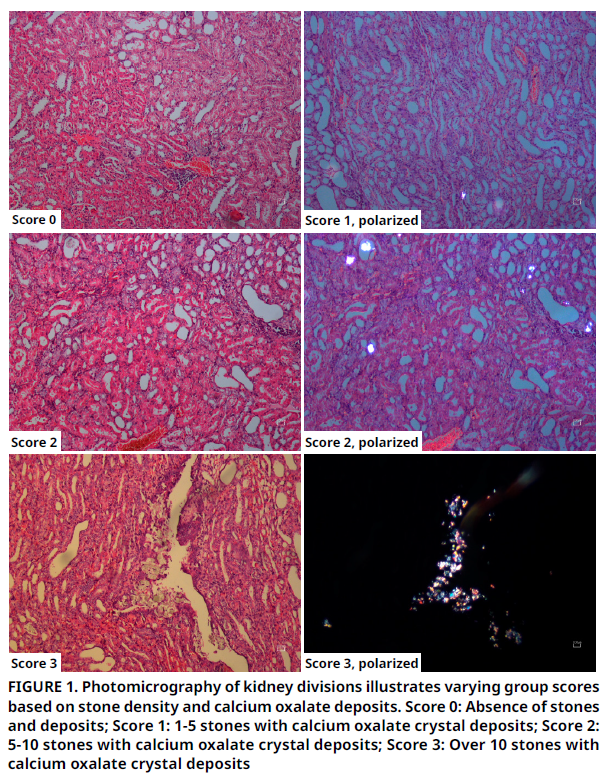
Este estudio tuvo como objetivo evaluar las propiedades antioxidantes y antiinflamatorias del extracto de metanol de Helichrysum plicatum DC. subsp. plicatum (EP) en el tracto urinario, utilizando un modelo de urolitiasis (U) inducido experimentalmente. El estudio incluyó cuatro grupos: al Grupo 1 se le administró una dieta estándar convencional, al Grupo 2 se le administró una dieta complementada con EP, al Grupo 3 se le administró una dieta estándar con urolitiasis inducida y el Grupo 4 recibió una dieta complementada con EP y con urolitiasis inducida. A las ratas de los Grupos 2 y 4 se les administró 500 mg·kg-1·dia-1 de EP mediante alimentación por sonda durante 21 días. La urolitiasis se indujo en los Grupos 3 y 4 mediante la administración de 1 % de etilenglicol y 1 % de cloruro de amonio en su agua potable durante 21 días para crear un modelo de urolitiasis por oxalato de calcio (CaOx). El estudio analizó las concentraciones plasmáticas de sustancias reactivas al ácido tiobarbitúrico (TBARS), un indicador de estrés oxidativo (EO), así como los niveles de EP en el suero. Además, se midieron los niveles de oxalato (Ox), urea, calcio y la depuración de creatinina en sangre y orina, y se realizaron evaluaciones histológicas de rutina. Los resultados indicaron concentraciones significativamente más altas de EP en los grupos que recibieron EP (P<0,001), mientras que las concentraciones plasmáticas de TBARS fueron más bajas en el Grupo 4 en comparación con el Grupo 3 (P=0,001). Los hallazgos sugieren que EP reduce el EO al disminuir los niveles plasmáticos de TBARS inducidos por CaOx, debido a sus propiedades antioxidantes y antiinflamatorias. Además, las mediciones bioquímicas realizadas respaldaron los efectos antiurolíticos de EP. En resumen, este estudio respalda la hipótesis de que las propiedades antioxidantes y antiinflamatorias de EP ayudan a prevenir el EO, que es un factor en la formación de cálculos, previniendo así el daño renal agudo y la formación de cálculos.
Descargas
Citas
Ilhan M, Ergene B, Süntar I, Özbilgin S, Saltan-Çitoğlu G, Demirel MA, Keleş H, Altun L, Küpeli-Akkol E. Preclinical evaluation of antiurolithiatic activity of Viburnum opulus L. on sodium oxalate-induced urolithiasis rat model. Evid. Based Compl. Alternat. Med. [Internet]. 2014; 2014:578103. doi: https://doi.org/f6dsz4 DOI: https://doi.org/10.1155/2014/578103
Araújo Viel T, Diogo Domingos C, da Silva Monteiro AP, Riggio Lima-Landman MT, Lapa AJ, Souccar C. Evaluation of the antiurolithiatic activity of the extract of Costus spiralis Roscoe in rats. J. Ethnopharmacol. [Internet]. 1994; 66(2):193–198. doi: https://doi.org/d8zr4v DOI: https://doi.org/10.1016/S0378-8741(98)00171-8
Atmani F, Farell G, Lieske JC. Extract from Herniaria hirsute coats calcium oxalate monohydrate crystals and blocks their adhesion to renal epithelial cells. J. Urol. [Internet]. 2004; 172(4):1510–1514. doi: https://doi.org/c4hzm3 DOI: https://doi.org/10.1097/01.ju.0000131004.03795.c5
Ramello A, Vitale C, Marangella M. Epidemiology of nephrolithiasis. J. Nephrol. 2000; 13(Suppl. 3):s45–s50. PMID:11132032
Sohgaura A, Bigoniya P. A Review on epidemiology and etiology of renal stone. Am. J. Drug Discov. Dev. [Internet]. 2017; 7(2):54-62. doi: https://doi.org/g8z65g DOI: https://doi.org/10.3923/ajdd.2017.54.62
Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol. Res. [Internet]. 2005; 33:349–357. doi: https://doi.org/bpzzn3 DOI: https://doi.org/10.1007/s00240-005-0492-4
Rodgers AL. Physicochemical mechanisms of stone formation. Urolithiasis [Internet]. 2017; 45:27–32. doi: https://doi.org/g8z65h DOI: https://doi.org/10.1007/s00240-016-0942-1
Hackett RL, Shevock PN, Khan SR. Madin-Darby canine kidney cells are injured by exposure to oxalate and to calcium oxalate crystals. Urol. Res. [Internet]. 1994; 22:197–203. doi: https://doi.org/cbzm6d DOI: https://doi.org/10.1007/BF00541892
Khan SR. Role of renal epithelial cells in the initiation of calcium oxalate stones. Nephron Exp. Nephrol. [Internet]. 2004; 98(2):e55–e60. doi: https://doi.org/cxq54x DOI: https://doi.org/10.1159/000080257
Khan SR. Pathogenesis of oxalate urolithiasis: Lessons from experimental studies with rats. Am. J. Kidney Dis. [Internet]. 1991; 17(4):398–401. doi: https://doi.org/g8z65j DOI: https://doi.org/10.1016/S0272-6386(12)80631-7
Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue culture studies. J. Clin. Exp. Nephrol. [Internet]. 2004; 8:75–88. doi: https://doi.org/bmhhn9 DOI: https://doi.org/10.1007/s10157-004-0292-0
Gurocak S, Kupeli B. Consumption of historical and current phytother- apeutic agents for urolithiasis: a critical review. J. Urol. [Internet]. 2006; 176(2):450–455. doi: https://doi.org/dfjnzm DOI: https://doi.org/10.1016/j.juro.2006.03.034
Grases F, Melero G, Costa-Bauza A, Prieto R, March JG. Urolithiasis and phytotherapy. Int. Urol. Nephrol. [Internet]. 1994; 26(5):507–511. doi: https://doi.org/fr97nt DOI: https://doi.org/10.1007/BF02767650
Khan SR, Kok DJ. Modulators of urinary stone formation. Front. Biosci. [Internet]. 2004; 9:1450–1482. doi: https://doi.org/bzvj7b DOI: https://doi.org/10.2741/1347
Yasui T, Okada A, Hamamoto S, Ando R, Taguchi K, Tozawa K, Kohri K. Pathophysiology-based treatment of urolithiasis. Int. J. Urol. [Internet]. 2017; 24(1):32–38. doi: https://doi.org/f9ndhj DOI: https://doi.org/10.1111/iju.13187
Zeng X, Xi Y, Jiang W. Protective roles of flavonoids and flavonoid-rich plant extracts against urolithiasis: A review. Crit. Rev. Food Sci. Nutr. [Internet]. 2018; 59(13):2125–2135. doi: https://doi.org/g8z65k DOI: https://doi.org/10.1080/10408398.2018.1439880
Facino RM, Carini M, Franzoi L, Pirola O, Bosisio E. Phytochemical characterization and radical scavenger activity of flavonoids from Helichrysum italicum G. Don (Compositae). Pharm. Res. [Internet]. 1990; 22(6):709–721. doi: https://doi.org/bcd6fd DOI: https://doi.org/10.1016/S1043-6618(05)80097-0
Binu TV, Vijayakumari B. Herbal plants as a remedy for urolithiasis - A review. Int. J. Pharm. Res. Dev. 2014; 6(6):35–39.
Altundag E, Ozturk M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia Soc. Behav. Sci. [Internet]. 2011; 19:756–777. doi: https://doi.org/b79m79 DOI: https://doi.org/10.1016/j.sbspro.2011.05.195
Aslan M, Orhan DD, Orhan N, Sezik E, Yesilada E. In vivo antidiabetic and antioxidant potential of Helichrysum plicatum ssp. plicatum capitulums in streptozotocin-induced-diabetic rats. J. Ethnopharmacol. [Internet]. 2007; 109(1):54–59. doi: https://doi.org/bjfw45 DOI: https://doi.org/10.1016/j.jep.2006.07.001
Ozbek T, Gulluce M, Adiguzel A, Ozkan H, Sahin F, Orhan F. Antimutagenic activity of the methanol extract of Helichrysum plicatum ssp plicatum. Asian J. Chem. [Internet]. 2009 [cited 12 Jul. 2024]; 21(4):2705–2710. Available in: https://goo.su/JsLi
Demir A, Mercanoglu Taban B, Aslan M, Yesilada E, Aytac SA. Antimicrobial effect of Helichrysum plicatum subsp plicatum. Pharm. Biol. [Internet]. 2009; 47(4):289–297. doi: https://doi.org/cgkwwp DOI: https://doi.org/10.1080/13880200802590434
Schinella GR, Tournier HA, Prieto JM, Mordujovich de Buschiazzo P, Rios JL. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. [Internet]. 2002; 70(9):1023–1033. doi: https://doi.org/fjm45w DOI: https://doi.org/10.1016/S0024-3205(01)01482-5
Aydin T. Secondary metabolites of Helichrysum plicatum DC. subsp. plicatum flowers as strong carbonic anhydrase, cholinesterase and α-glycosidase inhibitors. Z. Naturforsch. C. [Internet]. 2020; 75(5-6):153–159. doi: https://doi.org/g8z65m DOI: https://doi.org/10.1515/znc-2020-0026
Sahin K, Tuzcu M, Sahin N, Akdemir F, Ozercan I, Bayraktar S, Kucuk O. Inhibitory effects of combination of lycopene and genistein on 7,12- dimethyl benz(a)anthracene-induced breast cancer in rats. Nutr. Cancer. [Internet]. 2011; 63(8):1279–1286. doi: https://doi.org/bmjrkd DOI: https://doi.org/10.1080/01635581.2011.606955
Coskun H, Andic F, Daglioglu YK, Doran F, Sahin K, Tunali C, Kucuk O. Lycopene in the prevention of radiation-induced esophagitis. Nutr. Cancer. [Internet]. 2017; 69(2):319–329. doi: https://doi.org/g8z65n DOI: https://doi.org/10.1080/01635581.2017.1265133
Wazowicz W, Nève J, Peretz A. Optimized steps in fluorometric determination of acid-thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence of sample preservation and storage. Clin. Chem. [Internet]. 1993; 39(12):2522–2526. doi: https://doi.org/g8z65p DOI: https://doi.org/10.1093/clinchem/39.12.2522
Andic F, Garipagaoglu M, Yurdakonar E, Yurdakonar E, Tuncel M, Kucuk O. Lycopene in the prevention of gastrointestinal toxicity of radiotherapy. Nutr. Cancer. [Internet]. 2009; 61(6):784-788. doi: https://doi.org/bdx9xb DOI: https://doi.org/10.1080/01635580903285171
Oksay T, Yunusoğlu S, Calapoğlu M, Candan IA, Onaran I, Ergün O, Özorak A. Protective impact of resveratrol in experimental rat model of hyperoxaluria. Int. Urol. Nephrol. [Internet]. 2017; 49:769–775. doi: https://doi.org/f96tmc DOI: https://doi.org/10.1007/s11255-017-1534-x
Bayir Y, Halıcı Z, Keles MS, Colak S, Cakır A, Kaya Y, Akcay F. Helichrysum plicatum DC. subsp. plicatum extract as a preventive agent in experimentally induced urolithiasis model. J. Ethnopharmacol. [Internet]. 2011; 138(2):408–414. doi: https://doi.org/cq2wcw DOI: https://doi.org/10.1016/j.jep.2011.09.026
Onaran M, Orhan N, Farahvash A, Ekin HN, Kocabiyik M, Gönül II, Şen I, Aslan M. Successful treatment of sodium oxalate ınduced urolithiasis with Helichrysum flowers. J. Ethnopharmacol. [Internet]. 2016; 186:322–328. doi: https://doi.org/g8z65q DOI: https://doi.org/10.1016/j.jep.2016.04.003
Koul H, Kennington L, Nair G, Honeyman T, Menon M, Scheid C. Oxalate induced initiation of DNA synthesis in LLC-PK1 cells, a line of renal epithelial cells. Biochem. Biophys. Res. Comm. [Internet]. 1994; 205(3):1632–1637. doi: https://doi.org/ft9s4s DOI: https://doi.org/10.1006/bbrc.1994.2854
Scheid C, Koul H, Hill WA, Luber-Narod J, Kennington L, Honeyman T, Jonassen J, Menon M. Oxalate toxicity in LLC-PK1 cells: role of free radicals. Kidney Int. [Internet]. 1996; 49(2):413–419. doi: https://doi.org/bnqd3p DOI: https://doi.org/10.1038/ki.1996.60
Thamilselvan S, Hackett RL, Khan SR. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J. Urol. [Internet]. 1997; 157(3):1059–1063. doi: https://doi.org/b2k2vm DOI: https://doi.org/10.1016/S0022-5347(01)65141-3
Thamilselvan S, Byer KJ, Hackett RL, Khan SR. Free radical scavengers catalase and superoxide dismutase provide protection from oxalate associated injury to LLC-PK1 and MDCK cells. J. Urol. [Internet]. 2000; 164(1):224–229. doi: https://doi.org/ftzghm DOI: https://doi.org/10.1016/S0022-5347(05)67499-X
Thamilselvan S, Khan SR, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol. Res. [Internet]. 2003; 31:3–9. doi: https://doi.org/b4n7ff DOI: https://doi.org/10.1007/s00240-002-0286-x
Selvam R. Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol. Res. [Internet]. 2002; 30:35–47.doi: https://doi.org/c9b73x DOI: https://doi.org/10.1007/s00240-001-0228-z
Ilbey YO, Ozbek E, Simsek A, Cekmen M, Somay A, Tasci AI. Effects of pomegranate juice on hyperoxaluria-induced oxidative stress in the rat kidneys. Ren. Fail. [Internet]. 2009; 31(6):522–531. doi: https://doi.org/bxm8cr DOI: https://doi.org/10.1080/08860220902963871
Huang HS, Ma MC, Chen CF, Chen J. Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology [Internet]. 2003; 62(6):1123–1128. doi: https://doi.org/fqqkg5 DOI: https://doi.org/10.1016/S0090-4295(03)00764-7
Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P. Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Uro. Res. [Internet]. 2005; 33:65–69. doi: https://doi.org/br57zv DOI: https://doi.org/10.1007/s00240-004-0444-4
Ceban E, Banov P, Galescu A, Botnari V. Oxidative stress and antioxidant status in patients with complicated urolithiasis. J. Med. Life. [Internet]. 2016 [cited 18 Jul. 2024]; 9(3):259–262. PMID: 27974930. Available in: https://goo.su/5XI7n
Derechos de autor 2025 Selvinaz Yakan, Kıvılcım Eren Erdoğan, Yusuf Kenan Dağlıoğlu, Tuba Aydın, Ahmet Çakır

Esta obra está bajo licencia internacional Creative Commons Reconocimiento-NoComercial-CompartirIgual 4.0.


















