Potencial terapéutico de Ziziphus spina–christi: Un antiséptico natural con propiedades antioxidantes y antiinflamatorias
Resumen
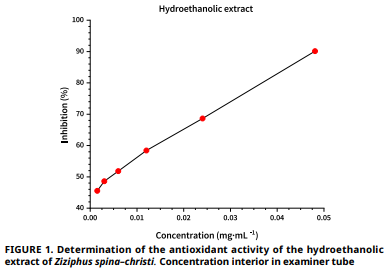
Ziziphus spina–christi, una planta medicinal ampliamente distribuida en Argelia y perteneciente a la familia Rhamnaceae, se utiliza tradicionalmente para tratar diversas dolencias debido a sus múltiples propiedades biológicas. Este estudio tiene como objetivo evaluar las actividades biológicas del extracto hidroetanólico de sus hojas, incluyendo propiedades antioxidantes, antibacterianas y cicatrizantes, además de cuantificar sus compuestos bioactivos para desarrollar una solución antiséptica natural. En un modelo experimental con ratas, se aplicaron distintas concentraciones del extracto (5, 10 y 15 %) y se compararon con controles positivo (Betaderme), negativo (sin tratamiento) y un grupo adicional control. También se evaluó la actividad antiinflamatoria mediante el modelo de edema ótico inducido por xileno, administrando dosis de 250, 400 y 600 mg·kg-1 y comparando sus efectos con el antiinflamatorio estándar NIFLUMENE (250 mg·kg-1). Los resultados mostraron una alta presencia de compuestos activos (CFT: 1,06 ± 0,03 mg QE·g-1; rendimiento de extracción: 13,6 %), una fuerte actividad antioxidante (CI₅₀: 0,004 ± 0,004 mg·mL-1) y una cicatrización significativamente acelerada con la solución al 10 % (96,25 ± 7,5 %), superior incluso a Betaderme. Además, la dosis de 400 mg·kg-1 presentó la mayor inhibición de inflamación (65,11 %). Estos hallazgos resaltan el potencial terapéutico de Ziziphus spina–christi como un eficaz agente antiséptico natural.
Descargas
Citas
Ye L, Jia Y, Ji K, Sanders AJ, Xue K, Ji J, Mason MD, Jiang WG. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. (Review). Oncol. lett. [Internet]. 2015; 10(3):1240–1250. doi: https://doi.org/gpdngx DOI: https://doi.org/10.3892/ol.2015.3459
Saad B, Zaid H, Shanak S, Kadan S. Introduction to medicinal plant safety and efficacy. In: Saad B, Zaid H, Shanak S, Kadan S, editors. Anti–diabetes and anti–obesity medicinal plants and phytochemicals. Safety, Efficacy, and Action Mechanisms [Internet]. Cham (Switzerland): Springer Cham. 2017; 21:55. doi. https://doi.org/qg9w DOI: https://doi.org/10.1007/978-3-319-54102-0_2
Rasool Hassan BA. Medicinal plants (importance and uses). Pharm. Anal. Acta. [Internet]. 2012; 3(10):2153–2435. doi: https://doi.org/qhcz DOI: https://doi.org/10.4172/2153-2435.1000e139
Bhushan P, Ashok DBV, Mukund C. Ayurveda and natural products drug discovery. Cur. Sci. [Internet]. 2004 [cited Mar. 5, 2025]; 86(6):789–799. Available in: https://goo.su/znEOyQz
Devkota HP, Paudel KR, Khanal S, Baral A, Panth N, Adhikari– Devkota A, Jha NK, Das N, Singh SK, Chellappan DK, Dua K, Hansbro PM. Stinging Nettle (Urtica dioica L.): nutritional composition, bioactive compounds, and food functional properties. Molecules [Internet]. 2022; 27(16):5219. doi: https://doi.org/qhc4 DOI: https://doi.org/10.3390/molecules27165219
Barnes J, Anderson LA, Phillipson JD. Herbal Medicines: A guide for healthcare professionals. 3rd ed. London (UK): Pharmaceutical Press. 2007.
Langer S, Salakdeh MS, Goertz O, Steinau H, Steinstraesser L, Homann H. The impact of topical antiseptics on skin microcirculation. Eur. J. Med. Res. [Internet]. 2004 [cited Oct. 30, 2025]; 9(9):449–454. PMID: 15546810. Available in: https://goo.su/XiSsu7j
Suliman MB, Mohammed AA. Preliminary phytochemical screening and antibacterial activity of ethanolic and aqueous extracts of Sudanese medicinal plant Ziziphus spinachristi L leaves. Arab. J. Med. Aromat. Plants. [Internet]. 2018; 4(1):36–44. doi: https://doi.org/qhc6
Almeer R S, Albasher G, Alotibi F, Alarifi S, Ali D, Alkahtani S. Ziziphus spina–christi leaf extract suppressed mercury chloride–induced nephrotoxicity via nrf2–antioxidant pathway activation and inhibition of inflammatory and apoptotic signaling. Oxid. Med. Cell. Longev. [Internet]. 2019; 2019:563468. doi: https://doi.org/qhc7 DOI: https://doi.org/10.1155/2019/5634685
Gebrehiwot H, Ensermu U, Dekebo A, Endale M, Nefo Duke T. In Vitro antibacterial and antioxidant activities, pharmacokinetics, and In Silico molecular docking study of phytochemicals from the roots of Ziziphus spina–christi. Biochem. Res. Int. [Internet]. 2024; 2024:7551813. doi: https://doi.org/qhc8 DOI: https://doi.org/10.1155/2024/7551813
Mostafa MAH, Khojah HMJ, Ohta T. Isolation and identification of novel selective antitumor constituents, sidrin and sidroside, from Zizyphus spina–christi. Saudi Pharm. J. [Internet]. 2023; 31(6):1019–1028. doi: https://doi.org/qhf8 DOI: https://doi.org/10.1016/j.jsps.2023.04.029
Asgarpanah J, Haghighat E. Phytochemistry and pharmacologic properties of Ziziphus spina christi (L.) Willd. Afr. J. Pharm. Pharmacol. [Internet]. 2012; 6(31):2332–2339. doi: https://doi.org/qhf9 DOI: https://doi.org/10.5897/AJPP12.509
Markham KR. Techniques of flavonoid identification. London (UK): Academic Press; 1982.
Bahorun T, Gressier B, Trotin F, Brunet C, Dine T, Luyckx M, Vasseur J, Cazin M, Cazin J, Pinkas M. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneimittelforschung. [Internet] 1996 [cited Oct. 30, 2025]; 46(11):1086–1089. PMID: 8955870. Available in: https://goo.su/oFQh
Gupta V, Mittal P, Bansal P, Khokra SL , Kaushik D. Pharmacological potential of Matricaria recutita – A review. Int. J. Pharm. Sci. Drug Res. [Internet]. 2010; 2(1):12–16. doi: https://doi.org/qhgd DOI: https://doi.org/10.25004/IJPSDR.2010.020102
Rajan DS, Rajkumar M, Kumarappan C, Kumar KS. Wound healing activity of an herbal ointment containing the leaf extract of Ziziphus mauritiana Lam. Afr. J. Pharm. Pharmacol. [Internet]. 2013; 7(4):98–103. doi: https://doi.org/qhgf DOI: https://doi.org/10.5897/AJPP12.795
Karim N, Khan I, Khan W, Khan I, Khan A, Halim SA, Khan H, Hussain J, Al–Harrasi A. Anti–nociceptive and anti– inflammatory activities of asparacosin a involve selective cyclooxygenase 2 and inflammatory cytokines inhibition: An in–vitro, in–vivo, and in–silico approach. Front. Immunol. [Internet]. 2019; 10:581. doi: https://doi.org/gm4998 DOI: https://doi.org/10.3389/fimmu.2019.00581
Pacheco NR, Pinto NdCC, Mello da Silva J, Mendes RdF, Costa JdCd, Aragão DMDO, Castañon MCMN, Scio E. Cecropia pachystachya: a species with expressive in vivo topical anti– inflammatory and in vitro antioxidant effects. BioMed Res. Int. [Internet]. 2014; 2014:301294. doi: https://doi.org/f6xvd6 DOI: https://doi.org/10.1155/2014/301294
Manouze H, Bouchatta O, Gadhi AC, Bennis M, Sokar Z, Ba–M’hamed S. Anti–inflammatory, antinociceptive, and antioxidant activities of methanol and aqueous extracts of Anacyclus pyrethrum roots. Front. Pharmacol. [Internet]. 2017; 8:598. doi: https://doi.org/qhgg DOI: https://doi.org/10.3389/fphar.2017.00598
Tarfa AN, Saab HA, Tata FY, Tarfa AN, Chukwu L, Aremu DO. Phytochemical screening of ethanol extract of Ziziphus spina–christi (leaf) and Euphorbia hirta (whole plant) and their analgesic activity in Wistar albino mice. Hum. Heal. Halal Metrics. [Internet]. 2024; 5(2):25–32. doi: https://doi.org/qhgh
Abu–Raghif AR, Jasim G A, Hanoon MM. Antiproliferative activity of Zizyphus spina christi leaves methanol extract against rhabdomyosarcoma (rd) cell line. Int. J. Pharm. Pharm. Sci. [Internet]. 2017; 9(2):279–282. doi: https://doi.org/qhgj DOI: https://doi.org/10.22159/ijpps.2017v9i2.15013
Salamatou M, Bayoï JR. Antimicrobial activity of extracts from Ziziphus mauritiana (Rhamnaceae) bark, leaf and fruit against Staphylococcus isolated from fish. J. Agr. Food Sci. Biotech. [Internet]. 2024; 2(3):258–266 doi: https://doi.org/qhgk DOI: https://doi.org/10.58985/jafsb.2024.v02i03.56
Saeed S, Hassan AF, Suliman A, Moustafa AEA, Alali F. Methanolic Leaves Extract of Ziziphus spina–christi Inhibits Cell Proliferation and Migration of HER2–Positive Breast Cancer via p38 MAPK Signaling Pathway. Int. J. Mol. Sci. [Internet]. 2025; 26(2):654. doi: https://doi.org/qhgm DOI: https://doi.org/10.3390/ijms26020654
Pradal D. Eco–Procédés D’extraction de Polyphénols Antioxydants à Partir D’un Co–Produit Agro–Alimentaire [dissertation on the Internet]. Villeneuve–d’Ascq (France): Lille 1 University; 2016 [cited Oct. 30, 2025]. 266 p. Available in: https://goo.su/Xc7zYi
Elaloui M, Essghaier B, Ghazghazi H, Chaouachi RD, Bahri S, Hamdi S, Nasr RB, Jemaa JB, Ammari Y, Laamouri A. LC–MS analysis and biological activities of Ziziphus spina–christi L. (Christ’s Thorn, Jujube) leaves from Tunisia oasis. J. Explor. Res. Pharmacol. [Internet]. 2022; 7(4):215–222. doi: https://doi.org/qhgn DOI: https://doi.org/10.14218/JERP.2022.00001
Elaloui M, Ammari Y, Ghazghazi H, Gorrab A, Laamouri A, Driouich Chaouachi R. Effect on Ziziphus jujuba Mill. fruit powders embedded on physicochemical properties, biological activities, and rheologic quality of cake. Food Sci. Nutr. [Internet]. 2023;11(6):2942–2955. doi: https://doi.org/qhgp DOI: https://doi.org/10.1002/fsn3.3276
El–Sawaf AK, El–Moslamy SH, Kamoun EA, Hossain K. Green synthesis of trimetallic CuO/Ag/ZnO nanocomposite using Ziziphus spina–christi plant extract: characterization, statistically experimental designs, and antimicrobial assessment. Sci. Rep. [Internet]. 2024; 14(1):19718. doi: https://doi.org/qhgq DOI: https://doi.org/10.1038/s41598-024-67579-5
Elhady SS, Goda MS, Mehanna ET, El–Sayed NM, Hazem RM, Elfaky MA, Almalki AJ, Mohamed MS, Abdelhameed RFA. Ziziphus spina–christi L. extract attenuates bleomycin– induced lung fibrosis in mice via regulating TGF–β1/ SMAD pathway: LC–MS/MS Metabolic profiling, chemical composition, and histology studies. Biomed. Pharmacother. [Internet]. 2024; 176:116823. doi: https://doi.org/qhgr DOI: https://doi.org/10.1016/j.biopha.2024.116823
Khaleel SM, Jaran AS, Haddadin MS. Evaluation of total phenolic content and antioxidant activity of three leaf extracts of Ziziphus spina–christi (Sedr) grown in Jordan. J. Adv. Med. Med. Res. [Internet]. 2016; 14(6):1–8. doi: https://doi.org/qhgs DOI: https://doi.org/10.9734/BJMMR/2016/24935
Al–Mutairi MH, Ali S, Aly SM, Aldebasi Y. Antibacterial activity of sider (Ziziphus spinachristi), leaves extract against selected pathogenic bacteria. Euro J. Pharm. Med. Res. [Internet]. 2016 [cited Oct. 30, 2025]; 3(5):138–144. Available in: https://goo.su/wHm8i
Okoli C, Ezike A, Akah P, Udegbunam S, Okoye T, Mbanu T, Ugwu E. Studies on wound healing and antiulcer activities of extract of aerial parts of Phyllanthus niruri L.(Euphorbiaceae). Am. J. Pharmacol. Toxicol. [Internet]. 2009; 4(4):118–126. doi: https://doi.org/d7d4pr DOI: https://doi.org/10.3844/ajptsp.2009.118.126
Malviya N, Jain S. Wound healing activity of aqueous extract of Radix paeoniae root. Acta Pol. Pharm. [Internet]. 2009 [cited Oct. 30, 2025]; 66(5):543–547. PMID: 19894650. Available in: https://goo.su/SOuRGr
Rotelli AE, Guardia T, Juárez AO, De la Rocha NE, Pelzer LE. Comparative study of flavonoids in experimental models of inflammation. Pharmacol. Res. [Internet]. 2003; 48(6):601–606. doi: https://doi.org/dwthx7 DOI: https://doi.org/10.1016/S1043-6618(03)00225-1
Sowemimo A, Samuel F, Fageyinbo M S. Anti–inflammatory activity of Markhamia tomentosa (Benth.) K. Schum. Ex Engl. ethanolic leaf extract. J. Ethnopharmacol. [Internet]. 2013; 149(1):191–194. doi: https://doi.org/f49dm2 DOI: https://doi.org/10.1016/j.jep.2013.06.020
Tong L, Chen T, Chen Z, Zhang P, Pi H, Ruan H, Wu J. Anti– inflammatory activity of omphalocarpin isolated from Radix Toddaliae Asiaticae. J. Ethnopharmacol. [Internet]. 2014; 155(3):1553–1560. doi: https://doi.org/f6mp63 DOI: https://doi.org/10.1016/j.jep.2014.07.055
Abdallah EM, Elsharkawy ER, Ed–dra A. Biological activities of methanolic leaf extract of Ziziphus mauritiana. Biosci. Biotech Res. Comm. [Internet]. 2016; 9(4): 605–614. doi: https://doi.org/qhgv DOI: https://doi.org/10.21786/bbrc/9.4/6
Abdullah, Khan MA, Ahmad W, Ahmad M, Adhikari A, Ibrar M, Rehman MU, Asif M. Antioxidant, antinociceptive, anti– inflammatory, and hepatoprotective activities of pentacyclic triterpenes isolated from Ziziphus oxyphylla Edgew. Drug Chem. Toxicol. [Internet]. 2022; 45(4):1796–1807. doi: https://doi.org/qhgw DOI: https://doi.org/10.1080/01480545.2021.1880427
Breinbauer R, Vetter IR, Waldmann H. From protein domains to drug candidates—natural products as guiding principles in the design and synthesis of compound libraries. Angew. Chem. Int. Ed. [Internet]. 2002; 41(16):2878–2890. doi: https://doi.org/b2twgk DOI: https://doi.org/10.1002/1521-3773(20020816)41:16<2878::AID-ANIE2878>3.0.CO;2-B
Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. [Internet]. 2007; 70(3):461–477. doi: https://doi.org/bzwphb DOI: https://doi.org/10.1021/np068054v