Detección del virus de PRRS en la mosca doméstica (Musca domestica) en una granja porcina en el sureste de México
Resumen
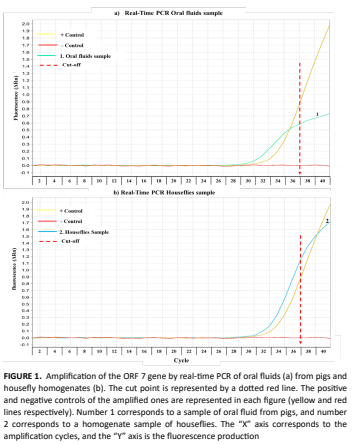
El objetivo de este estudio fue detectar la presencia del virus del síndrome respiratorio y reproductivo porcino en moscas domésticas (Musca domestica) en una granja comercial de cerdos. Para conocer la circulación del virus del síndrome respiratorio y reproductivo porcino en la granja, se tomaron muestras de sangre de 1277 y 26 muestras de fluidos orales de cerdos alojados en corrales para detectar el ácido nucleico d el virus del síndrome respiratorio y reproductivo porcino utilizando la prueba de RT-PCR en tiempo real. Se recolectaron 300 moscas, utilizando una red entomológica, y se formaron 50 grupos de 6 moscas cada una. Los datos se analizaron mediante estadísticas descriptivas. De los sueros analizados el 95,74% (1224/1277) fueron positivos en la prueba ELISA. El ácido nucleico del virus del síndrome respiratorio y reproductivo porcino se detectó en el 34,6% (9/26) de los fluidos orales y en el 4% (2/50) de las muestras de moscas analizadas. La prueba de PCR en tiempo real permitió detectar el virus del síndrome respiratorio y reproductivo porcino en moscas domésticas. En conclusión, más estudios de secuenciación del virus son necesarios para comprender mejor la función de las moscas en la transmisión del síndrome respiratorio y reproductivo porcino.
Descargas
Citas
Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green Al, Zimmerman JJ. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. [Internet]. 2005; 227(3):385-392. doi: https://doi.org/fjmgq3 DOI: https://doi.org/10.2460/javma.2005.227.385
Holtkamp DJ, Kliebenstein JB, Neumann EJ, Zimmerman JJ, Rotto HF, Yoder TK, Wang C, Yeske PE, Mowrer CL, Haley CA. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J. Swine Health Prod. [Internet]. 2013 [cited 22 May 2025]; 21(2):72-84. Available in: https://goo.su/UCJXvn DOI: https://doi.org/10.54846/jshap/754
Taira O, Kato A, Tsutsumi N, Sugiura K. Epidemiological review of porcine reproductive and respiratory syndrome virus (PRRSV) in Japan: From Discovery and Spread to Economic Losses and Future Prospects. Vet. Sci. [Internet]. 2025; 12(6):554. doi: https://doi.org/g9vrqq DOI: https://doi.org/10.3390/vetsci12060554
Cho JG, Dee SA. Porcine reproductive and respiratory syndrome virus. Theriogenology. [Internet]. 2006; 66(3):655-662. doi: https://doi.org/ctkxs6 DOI: https://doi.org/10.1016/j.theriogenology.2006.04.024
Wensvoort G, de Kluyver EP, Luijtze EA, den Besten A, Harris L, Collins JE, Christianson WT, Chladek D. Antigenic comparison of Lelystad virus and swine infertility and respiratory syndrome (SIRS) virus. J. Vet. Diagn. Investig. [Internet]. 1992; 4(2):134-138. doi: https://doi.org/fw8k5v DOI: https://doi.org/10.1177/104063879200400203
Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS, Gorcyca D, Chladek D. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Investig. [Internet]. 1992; 4(2):117-126. doi: https://doi.org/ cwjw9z DOI: https://doi.org/10.1177/104063879200400201
Allende R, Lewis TL, Lu Z, Rock DL, Kutish GF, Ali A, Doster AR, Osorio FA. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J. Gen. Virol. [Internet]. 1999; 80(2):307-315. doi: https://doi.org/p8f2 DOI: https://doi.org/10.1099/0022-1317-80-2-307
Batista L, Pijoan C, Torremorell M. Experimental injection of gilts with porcine reproductive and respiratory syndrome virus (PRRSV) during acclimatization. J. Swine Health Prod. [Internet]. 2002; 10(4):147-150. doi: https://doi.org/p8f3 DOI: https://doi.org/10.54846/jshap/335
Batista L, Pijoan C, Dee S, Olin M, Molitor T, Joo HS, Xiao Z, Murtaugh M. Virological and immunological responses to porcine reproductive and respiratory syndrome virus in a large population of gilts. Can. J. Vet. Res. [Internet]. 2004 [cited 12 May 2025]; 68(4):267-273. Available in: https://goo.su/PLo0qI
Fano E., Olea L, Pijoan C. Eradication of porcine reproductive and respiratory syndrome virus by serum inoculation of naïve gilts. Can. J. Vet. Res. [Internet]. 2005 [cited 12 Feb 2025]; 69(1):71–74. Available in: https://goo.su/x7JkS
Otake S, Dee SA, Moon RD, Rossow KD, Trincado C, Farnham M, Pijoan C. Survival of porcine reproductive and respiratory syndrome virus in houseflies. Can. J. Vet. Res. [Internet]. 2003 [cited 30 May 2025]; 67(3):198-203. Available in: https://goo.su/oyZGY
Fasanella A., Scasciamacchia S, Garofolo G, Giangaspero A, Tarsitano E, Adone R. Evaluation of the house fly Musca domestica as a mechanical vector for an anthrax. PLoS One. [Internet]. 2010; 5(8):e12219. doi: https://doi.org/c3xwbf DOI: https://doi.org/10.1371/journal.pone.0012219
Hung KY, Michailides TJ, Millar JG, Wayadande A, Gerry AC. House fly (Musca domestica L.) attraction to insect honeydew. PLoS One. [Internet]. 2015; 10(5):e0124746. doi: https://doi.org/f7kx7j DOI: https://doi.org/10.1371/journal.pone.0124746
Dee SA, Schurrer JA, Moon RD, Fano E, Trincado C, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus under field conditions during a putative increase in the fly population. J. Swine Health Prod. [Internet]. 2004; 12(5):242-245. doi: https://doi.org/p8g2 DOI: https://doi.org/10.54846/jshap/414
Schurrer JA, Dee SA, Moon RD, Rossow KD, Mahlum C, Mondaca E, Otake S, Fano E, Collins JE, Pijoan C. Spatial dispersal of porcine reproductive and respiratorysyndrome virus-contaminated flies after contact with experimentally infected pigs. Am. J. Vet. Res. [Internet]. 2004; 65(9):1284-1292. doi: https://doi.org/cnz6fr DOI: https://doi.org/10.2460/ajvr.2004.65.1284
Schurrer JA, Dee SA, Moon RD, Murtaugh MP, Finnegan CP, Deen J, Kleiboeker SB, Pijoan CBJ. Retention of ingested porcine reproductive and respiratory syndrome virus in houseflies. Am. J. Vet. Res. [Internet]. 2005; 66(9):1517-1525. doi: https://doi.org/c3g82f DOI: https://doi.org/10.2460/ajvr.2005.66.1517
Instituto Nacional de Estadística y Geografía (INEGI). Prontuario de información geográfica municipal de los Estados Unidos Mexicanos. INEGI. [Internet]. 2009 [cited 10 Jun 2025]; 2:1-9 Available in: https://goo.su/GxP2QU
Holtkamp DJ, Polson DD, Torremorell M, Morrison B, Classen DM, Becton L, Henry S, Rodibaugh MT, Rowland RR, Snelson H, Straw B, Yeske P, Zimmerman J. Terminology for classifying siwne herd by porcine reproductive and respiratory syndrome virus status. J. Swine Health Prod. [Internet]. 2011; 19(1):44-56. doi: https://doi.org/p8g6 DOI: https://doi.org/10.54846/jshap/669
Prickett J, Simer R, Christopher-Hennings J, Yoon K, Evans RB, Zimmerman JJ. Detection of porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions. J. Vet. Diagn. Investig. [Internet]. 2008; 20(2):156-163. doi: https://doi.org/d3xqnm DOI: https://doi.org/10.1177/104063870802000203
Prickett J, Kim W, Simer R, Yoon K, Zimmerman J. Oral-fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections. J. Swine Health Prod. [Internet]. 2008; 16(2):86–91. doi: https://doi.org/p8g7 DOI: https://doi.org/10.54846/jshap/565
Roulston TH, Smith SA, Brewster AL. A comparison of pan trap and intensive net sampling techniques for documenting a bee (Hymenoptera: Apiformes) fauna. J. Kans. Entomol. Soc. [Internet]. 2007; 80(2):179-181. doi: https://doi.org/csft2w DOI: https://doi.org/10.2317/0022-8567(2007)80[179:ACOPTA]2.0.CO;2
Ramírez-Freire L, Alanís-Flores G, Ayala-Barajas R, Velazco-Macias C, Favela-Lara S. El uso de platos trampa y red entomológica en la captura de abejas nativas en el estado de Nuevo León, México. Acta Zool. Mex. [Internet]. 2014 [cited 15 May 2025]; 30(3):508-538. Available in: https://goo.su/7Q7v0sn DOI: https://doi.org/10.21829/azm.2014.30375
Otake S, Dee SA, Moon RD, Rossow KD, Trincado C, Pijoan C. Studies on the carriage and transmission of porcine reproductive and respiratory syndrome virus by individual houseflies (Musca domestica). Vet. Rec. [Internet]. 2004; 154(3):80-85. doi: https://doi.org/cjdd8k DOI: https://doi.org/10.1136/vr.154.3.80
Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): An overview. Vet. Microbiol. [Internet]. 1997; 55(1-4):309-316. doi: https://doi.org/fnxcr9 DOI: https://doi.org/10.1016/S0378-1135(96)01322-3
Nodelijk G, van Leengoed LAMG, Schoevers EJ, Kroese AH, De Jong MCM, Wensvoort G, Verheijden JHM. Seroprevalence of porcine reproductive and respiratory syndrome virus in Dutch weaning pigs. Vet. Microbiol. [Internet]. 1997; 56(1-2):21-32. doi: https://doi.org/bftz75 DOI: https://doi.org/10.1016/S0378-1135(96)01349-1
Geldhof MF, Van Breedam W, De Jong E, Lopez-Rodriguez A, Karniychuk UU, Vanhee M, Van Doorsselaere J, Maes D, Nauwynk HJ. Antibody response and maternal immunity upon boosting PRRSV-immune sows with experimental farm-specific and commercial PRRSV vaccines. Vet Microbiol. [Internet]. 2013; 167(3-4):260-271. doi: https://doi.org/f5qrv7 DOI: https://doi.org/10.1016/j.vetmic.2013.08.017
Flores-Mendoza L, Hernández J. Vacunas contra el virus del síndrome reproductivo y respiratorio porcino (PRRSV): escribiendo una historia. Vet. Mex. [Internet]. 2010 [cited 10 Jun 2025]; 41(2):139-159. Available in: https://goo.su/RaJUlR1
Nelson EA, Christopher-Hennings J, Benfield DA. Serum immune responses to the proteins of porcine reproductive and respiratory syndrome (PRRS) virus. J. Vet. Diagn. Investig. [Internet]. 1994; 6(4):410–415. doi: https://doi.org/dtxqhg DOI: https://doi.org/10.1177/104063879400600402
Li X, Galliher-Beckley A, Pappan L, Trible B, Kerrigan M, Beck A, Hesse R, Blecha F, Nietfeld JC, Rowland RR, Shi J. Comparison of host immune responses to homologous and heterologous type II porcine reproductive and respiratory syndrome virus (PRRSV) challenge in vaccinated and unvaccinated pigs. Biomed. Res. Int. [Internet]. 2014; 2014:416727. doi: https://doi.org/gb83qv DOI: https://doi.org/10.1155/2014/416727
De Regge N, Cay B. Comparison of PRRSV nucleic acid and antibody detection in pen-based oral fluid and individual serum samples in three different age categories of postweaning pigs from endemically infected farms. PLoS One. [Internet]. 2016; 11(11):e0166300. doi: https://doi.org/f9rj2r DOI: https://doi.org/10.1371/journal.pone.0166300
Olsen C, Karriker L, Wang C, Binjawadagi B, Renukaradhya G, Kittawornrat A, Lizano S, Coetzee J, Main R, Meiszberg A, Panyasing Y, Zimmerman J. Effect of collection material and sample processing on pig oral fluid testing results. Vet. J. [Internet]. 2013; 198(1):158-163. doi: https://doi.org/f5j6qg DOI: https://doi.org/10.1016/j.tvjl.2013.06.014
Seddon YM, Guy JH, Edwards SA. Optimising oral fluid collection from groups of pigs: effect of housing system and provision of ropes. Vet. J. [Internet]. 2012; 193(1):180-184. doi: https://doi.org/b6gx89 DOI: https://doi.org/10.1016/j.tvjl.2011.11.008
Prickett JR, Cutler S, Kinyon JM, Naberhaus N, Stensland WR, YooK, Zimmerman JJ. Stability of porcine reproductive and respiratory syndrome virus and antibody in swine oral fluid. J. Swine Health Prod. [Internet]. 2010 [cited 10 Jun 2025]; 18(4):187–195. Available in: https://goo.su/tZ1D5N DOI: https://doi.org/10.54846/jshap/645
Decorte I, Van der Stede Y, Nauwynck H, De Regge N, Cay AB. Effect of saliva stabilisers on detection of porcine reproductive and respiratory syndrome virus in oral fluid by quantitative reverse transcriptase real-time PCR. Vet. J. [Internet]. 2013; 197(2):224-228. doi: https://doi.org/f5chnp DOI: https://doi.org/10.1016/j.tvjl.2013.02.001
Wang YC, Chang YC, Chuang HL, Chiu CC, Yeh KS, Chang CC, Hsuan SL, Lin WH, Chen TH. Transmission of Salmonella between swine farms by the housefly (Musca domestica). J. Food Prot. [Internet]. 2011; 74(6):10121016. doi: https://doi.org/d79tmb DOI: https://doi.org/10.4315/0362-028X.JFP-10-394
Murvosh CM, Thaggard CW. Ecological studies of the house fly. Ann. Entomol. Soc. Am. [Internet]. 1996; 59(3):533-547. doi: https://doi.org/p8g8 DOI: https://doi.org/10.1093/aesa/59.3.533
Otake S, Dee SA, Rossow KD, Moon RD, Pijoan C. Mechanical transmission of porcine reproductive and respiratory syndrome virus by mosquitoes, Aedes vexans (Meigen). Can. J. Vet. Res. [Internet]. 2002 [cited 25 Jun 2025]; 66(3):191-195. Available in: https://goo.su/zx6HDn
Förster M, Klimpel S, Mehlhorn H, Sievert K, Messler S, Pfeffer K. Pilot study on synanthropic flies (e.g. Musca, Sarcophaga, Calliphora, Fannia, Lucilia, Stomoxys) as vectors of pathogenic microorganisms. Parasitol. Res. [Internet]. 2007; 101(1):243-246. doi: https://doi.org/fkrkm8 DOI: https://doi.org/10.1007/s00436-007-0522-y
Yoon KJ, Zimmerman JJ, Chang CC, Cancel-Tirado S, Harmon KM, McGinley MJ. Effect of challenge dose and route on porcine reproductive and respiratory syndrome virus (PRRSV) infection in young swine. Vet. Res. [Internet]. 1999 [cited 15 Jun 2025]; 30(6):629-638. Available in: https://goo.su/lRHer
Hernández A, Martín Vasallo P, Torres A, Salido E. Análisis del RNA: Estudio de la expresión génica. Nefrología. [Internet]. 1994 [cited 10 Jun 2025]; 14(2):145-162. Available in: https://goo.su/pkqyh
Morgan-Morrow WE, Roberts J. PRRS fact sheet for animal science. Anim. Sci. Facts. [Internet]. 2001 [cited 10 Jun 2025]; 817S:1-2. Available in: https://goo.su/UksMkj
Pitkin A, Deen J, Otake S, Moon R, Dee S. Further assessment of houseflies (Musca domestica) as vectors for the mechanical transport and transmission of porcine reproductive and respiratory syndrome virus under field conditions. Can. J. Vet. Res. [Internet]. 2009 [cited 10 Jun 2025]; 73(2):91-96. Available in: https://goo.su/zIA57L
Benfield DA, Nelson E, Collins JE, Harris L, Goyal SM, Robison D, Christianson WT, Morrison RB, Gorcyca D, Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J. Vet. Diagn. Investig. [Internet]. 1992; 4(2):127-133. doi: https://doi.org/dwrr3m DOI: https://doi.org/10.1177/104063879200400202
Grübel P, Hoffman JS, Chong FK, Burstein NA, Mepani C, Cave DR. Vector potential of houseflies (Musca domestica) for Helicobacter pylori. J. Clin. Microbiol. [Internet]. 1997; 35(6):1300-1303. doi: https://doi.org/ghszj4 DOI: https://doi.org/10.1128/jcm.35.6.1300-1303.1997
Bloemraad M, de Kluijver EP, Petersen A, Burkhardt GE, Wensvoort G. Porcine reproductive and respiratory syndrome: temperature and pH stability of Lelystad virus and its survival in tissue specimens from viraemic pigs. Vet. Microbiol. [Internet]. 1994; 42(4):361-371. doi: https://doi.org/fqmr38 DOI: https://doi.org/10.1016/0378-1135(94)90067-1
Tousignant SJ, Perez AM, Lowe JF, Yeske PE, Morrison RB. Temporal and spatial dynamics of porcine reproductive and respiratory syndrome virus infection in the United States. Am. J. Vet. Res. [Internet]. 2015; 76(1):70-76. doi: https://doi.org/f65j5v DOI: https://doi.org/10.2460/ajvr.76.1.70