Determinación de cepas de Enterococcus resistentes a la ampicilina, resistencia a antibióticos y genes de virulencia en muestras de leche y carne de pollo de la provincia de Sivas, Turquía
Resumen
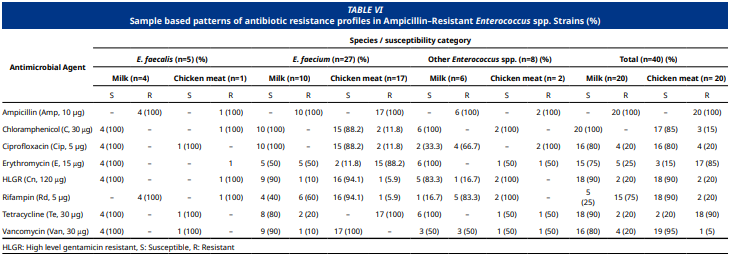
El objetivo de este estudio fue analizar la presencia de Enterococcus spp. resistentes a la ampicilina aislados en muestras de leche cruda y carne de pollo (Gallus gallus domesticus), centrándose en sus perfiles de resistencia antimicrobiana (AmpR) y genes de virulencia de la provincia de Sivas, Turquía. Se recolectaron y analizaron 210 muestras de leche cruda (n = 150; leche de vaca (Bos taurus), oveja (Ovis aries) y búfala (Bubalus bubalis)) y piezas frescas de pollo (n = 60; muslos y alas con piel). Se realizaron pruebas de susceptibilidad antimicrobiana mediante el método de difusión en disco, mientras que las concentraciones mínimas inhibitorias para los aislados de resistencia a la ampicilina se determinaron mediante microdilución en caldo. La reacción en cadena de la PCR identificó los aislados a nivel de especie y analizó los genes de virulencia clave (asa1, cylA, esp, gelE e hyl). Se aislaron 40 cepas Enterococcus spp. AmpRa partir de muestras de leche cruda y carne de pollo. De los aislados de leche cruda y carne de pollo, el 67,5 % se identificó como E. faecium, el 12,5 % como E. faecalis y el 20 % como otras especies de Enterococcus. Entre los aislados de resistencia a la ampicilina Enterococcus spp., se detectaron valores de concentraciones mínimas inhibitorias ≥ 16 μg·mL-1 en el 25,0 % de los aislados de leche y el 30,0 % de los aislados de muestras de carne de pollo. La difusión en disco reveló resistencia variada entre los aislados, con las más altas, la eritromicina (55,0%) y tetraciclina (50,0 %), seguida de rifampicina (42,5 %), ciprofloxacino (20,0 %), vancomicina (12,5%), gentamicina (10,0%) y cloranfenicol (7,5%). Los aislados de AmpR Enterococcus spp. exhibieron una tasa de resistencia a múltiples fármacos del 67,5 %. El análisis de genes de virulencia indicó la presencia del gen asa1 en un solo aislado de faecalis (2,5 %), mientras que no se detectaron los genes cylA, esp, gelE ni hyl. La detección de Enterococcus spp. resistentes a antibióticos en leche cruda y carne de pollo, así como la presencia de enterococos resistentes a la vancomicina y la gentamicina, son importantes para la salud pública. El monitoreo de enterococos resistentes a antimicrobianos en alimentos de origen animal es crucial en el marco del concepto “Una Salud”.
Descargas
Citas
Aamodt H, Mohn SC, Maselle S, Manji KP, Willems R, Jureen R, Langeland N, Blomberg B. Genetic relatedness and risk factor analysis of ampicillin–resistant and high–level gentamicin– resistant enterococci causing bloodstream infections in Tanzanian children. BMC Infect. Dis. [Internet]. 2015; 15:1-9. doi: https://doi.org/gb324s DOI: https://doi.org/10.1186/s12879-015-0845-8
Hollenbeck BL, Rice LB. Intrinsic and acquired resistance mechanisms in Enterococcus. Virulence [Internet]. 2012; 3(5):421-433. doi: https://doi.org/gnhw4f DOI: https://doi.org/10.4161/viru.21282
European Food Safety Authority (EFSA), European Centre for Disease Prevention and Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021- 2022. EFSA J. [Internet]. 2024; 22(2):e08583. doi: https://doi.org/nr2j DOI: https://doi.org/10.2903/j.efsa.2024.8583
Zhang Y, Du M, Chang Y, Chen LA, Zhang Q. Incidence, clinical characteristics, and outcomes of nosocomial Enterococcus spp. bloodstream infections in a tertiary–care hospital in Beijing, China: a four–year retrospective study. Antimicrob. Resist. Infect. Control. [Internet]. 2017; 6(1):1-11. doi: https://doi.org/px3b DOI: https://doi.org/10.1186/s13756-017-0231-y
Golob M, Pate M, Kušar D, Dermota U, Avberšek J, Papić B, Zdovc I. Antimicrobial resistance and virulence genes in Enterococcus faecium and Enterococcus faecalis from humans and retail red meat. Biomed. Res. Int. [Internet]. 2019; 2019:2815279. doi: https://doi.org/gm3wv5 DOI: https://doi.org/10.1155/2019/2815279
Mundy LM, Sahm DF, Gilmore M. Relationships between enterococcal virulence and antimicrobial resistance. Clin. Microbiol. Rev. [Internet]. 2000; 13(4):513-522. doi: https://doi.org/gph9ff DOI: https://doi.org/10.1128/CMR.13.4.513
Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert. Rev. Anti. Infect. Ther. [Internet]. 2014; 12(10):1221-1236. doi: https://doi.org/f6627h DOI: https://doi.org/10.1586/14787210.2014.956092
Willems RJ, Van Schaik W. Transition of Enterococcus faecium from commensal organism to nosocomial pathogen. Future Microbiol. [Internet]. 2009; 4(9):1125-1135. doi: https://doi.org/cdwh4f DOI: https://doi.org/10.2217/fmb.09.82
Hammerum AM, Lester CH, Heuer OE. Antimicrobial–resistant enterococci in animals and meat: a human health hazard?. Foodborne Pathog. Dis. [Internet]. 2010; 7(10):1137-1146. doi: https://doi.org/d8fpbz DOI: https://doi.org/10.1089/fpd.2010.0552
Willems RJ, Top J, Van Santen M, Robinson DA, Coque TM, Baquero F, Bonten MJ. Global spread of vancomycin–resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg. Infect. Dis. [Internet]. 2005; 11(6):821- 828. doi: https://doi.org/px3c DOI: https://doi.org/10.3201/eid1106.041204
Heikens E, Singh KV, Jacques–Palaz KD, van Luit–Asbroek M, Oostdijk EA, Bonten MJ, Murray BE, Willems RJ. Contribution of the enterococcal surface protein esp to pathogenesis of Enterococcus faecium endocarditis. Microbes Infect. [Internet]. 2011; 13(14-15):1185-1190. doi: https://doi.org/ddph9k DOI: https://doi.org/10.1016/j.micinf.2011.08.006
Sørensen TL, Blom M, Monnet DL, Frimodt–Møller N, Poulsen RL, Espersen F. Transient intestinal carriage after ingestion of antibiotic–resistant Enterococcus faecium from chicken and pork. N. Engl. J. Med. [Internet]. 2001; 345(16):1161-1166. doi: https://doi.org/bggvbt DOI: https://doi.org/10.1056/NEJMoa010692
Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. [Internet]. 2012; 10(4):266-278. doi: https://doi.org/f4nn43 DOI: https://doi.org/10.1038/nrmicro2761
Teixeira LM, Carvalho MDGS, Facklam RR, Shewmaker PL. Enterococcus. In: Jorgensen JH, Carroll KC, Funke G, Pfaller MA, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical Microbiology. 11th. ed. Washington, DC (USA): ASM Press; 2015. p. 403-421. DOI: https://doi.org/10.1128/9781555817381.ch23
Guzman–Prieto AM, van Schaik W, Rogers MR, Coque TM, Baquero F, Corander J, Willems RJ. Global emergence and dissemination of enterococci as nosocomial pathogens: attack of the clones?. Front. Microbiol. [Internet]. 2016; 7:788. doi: https://doi.org/ggdgkn DOI: https://doi.org/10.3389/fmicb.2016.00788
Lebreton F, Willems RJL, Gilmore MS. Enterococcus diversity, origins in nature, and gut colonization. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: From commensals to leading causes of drug resistant infection [Internet]. Boston (USA): Massachusetts Eye and Ear Infirmary; 2014 [cite Jun. 25, 2025]. p. 1-46. Available in: https://goo.su/yIORwV
Pesavento G, Calonico C, Ducci B, Magnanini A, Lo Nostro A. Prevalence and antibiotic resistance of Enterococcus spp. isolated from retail cheese, ready–to–eat salads, ham, and raw meat. Food Microbiol. [Internet]. 2014; 41:1-7. doi: https://doi.org/px3f DOI: https://doi.org/10.1016/j.fm.2014.01.008
Ribeiro J, Silva V, Monteiro A, Vieira–Pinto M, Igrejas G, Reis FS, Barros L, Poeta P. Antibiotic resistance among gastrointestinal bacteria in broilers: a review focused on Enterococcus spp. and Escherichia coli. Animals [Internet]. 2023; 13(8):1362. doi: https://doi.org/px3g DOI: https://doi.org/10.3390/ani13081362
Çetinkaya F, Elal–Muş T. Yararları ve Riskleriyle Gıda Kaynaklı Enterokoklar. J. Res. Vet. Med. [Internet]. 2010 [cited Jun 14, 2025]; 29(1): 77-83. Available in: https://goo.su/pJzJp
Kürekci C, Önen SP, Yipel M, Aslantaş Ö, Gündoğdu A. Characterisation of phenotypic and genotypic antibiotic resistance profile of enterococci from cheeses in Turkey. Korean J. Food Sci. Anim. Resour. [Internet]. 2016; 36(3):352-358. doi: https://doi.org/px3h DOI: https://doi.org/10.5851/kosfa.2016.36.3.352
Yılmaz EŞ, Aslantaş Ö, Önen SP, Türkyılmaz S, Kürekci C. Prevalence, antimicrobial resistance and virulence traits in enterococci from food of animal origin in Turkey. LWT Food Sci. Technol. [Internet]. 2016; 66:20-26. doi: https://doi.org/px3j DOI: https://doi.org/10.1016/j.lwt.2015.10.009
Sanlibaba P, Tezel BU, Senturk E. Antimicrobial resistance of Enterococcus species isolated from chicken in Turkey. Korean J. Food Sci. Anim. Resour. [Internet]. 2018; 38(2):391-402. doi: https://doi.org/g36pjz
DTU National Food Institute. Protocol for PCR amplification of E. faecium and E. faecalis recommended by the EURL–AR. 3rd version. [Internet]. Lyngby (Denmark): European Union Reference Laboratory for Antimicrobial Resistance; 2014 [cited Jan 24, 2023]. Available in: https://goo.su/SgfbJI
Ke D, Picard FJ, Martineau F, Ménard C, Roy PH, Ouellette M, Bergeron MG. Development of a PCR assay for rapid detection of enterococci. J. Clin. Microbiol. [Internet]. 1999; 37(11):3497-3503. doi: https://doi.org/px3k DOI: https://doi.org/10.1128/JCM.37.11.3497-3503.1999
Kariyama R, Mitsuhata R, Chow JW, Clewell DB, Kumon H. Simple and reliable multiplex PCR assay for surveillance isolates of vancomycin–resistant enterococci. J. Clin. Microbiol. [Internet]. 2000; 38(8):3092-3095. doi: https://doi.org/px3m DOI: https://doi.org/10.1128/JCM.38.8.3092-3095.2000
Cheng S, McCleskey FK, Gress MJ, Petroziello JM, Liu R, Namdari H, Salmen A, DelVecchio VG. A PCR assay for identification of Enterococcus faecium. J. Clin. Microbiol. [Internet]. 1997; 35(5):1248-1250. doi: https://doi.org/px3n DOI: https://doi.org/10.1128/jcm.35.5.1248-1250.1997
Vankerckhoven V, Van Autgaerden T, Vael C, Lammens C, Chapelle S, Rossi R, Jabes D, Goossens H. Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J. Clin. Microbiol. [Internet]. 2004; 42(10):4473-4479. doi: https://doi.org/fwtgjb DOI: https://doi.org/10.1128/JCM.42.10.4473-4479.2004
European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 8.1. [Internet]. European Committee on Antimicrobial Susceptibility Testing; 2018. [cited Jul. 3, 2024]. Available in: https://goo.su/QKidu15
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 30th. ed. Wayne (Pennsylvania, USA): Clinical and Laboratory Standards Institute; 2020.
Amuasi GR, Dsani E, Owusu–Nyantakyi C, Owusu FA, Mohktar Q, Nilsson P, Adu N, Hendriksen RS, Egyir B. Enterococcus species: insights into antimicrobial resistance and whole– genome features of isolates recovered from livestock and raw meat in Ghana. Front. Microbiol. [Internet]. 2023; 14:1254896. doi: https://doi.org/px3p DOI: https://doi.org/10.3389/fmicb.2023.1254896
Gajewska J, Chajęcka–Wierzchowska W, Byczkowska– Rostkowska Z, Saki M. Biofilm formation capacity and presence of virulence determinants among Enterococcus species from milk and raw milk cheeses. Life [Internet]. 2023; 13(2):495. doi: https://doi.org/px3q DOI: https://doi.org/10.3390/life13020495
Morandi S, Silvetti T, Lopreiato V, Piccioli–Cappelli F, Trevisi E, Brasca M. Biodiversity and antibiotic resistance profile provide new evidence for a different origin of enterococci in bovine raw milk and feces. Food Microbiol. [Internet]. 2024; 120:104492. https://doi.org/px3r DOI: https://doi.org/10.1016/j.fm.2024.104492
Inhoque–Pereira R, Prichula J, Aguiar–Santestevan N, Alves–D’Azevedo P, de Souza–Motta A, Guedes–Frazzon AP. Virulence profiles in Enterococcus spp. isolated from raw buffalo’s milk in South Brazil. Res. J. Microbiol. [Internet]. 2017; 12(4):248-254. doi: https://doi.org/px3s DOI: https://doi.org/10.3923/jm.2017.248.254
Kim HJ, Youn HY, Kang HJ, Moon JS, Jang YS, Song KY, Seo KH. Prevalence and virulence characteristics of Enterococcus faecalis and Enterococcus faecium in bovine mastitis milk compared to bovine normal raw milk in South Korea. Animals [Internet]. 2022; 12(11):1407. doi: https://doi.org/px3t DOI: https://doi.org/10.3390/ani12111407
Buzatto–de Souza D, Inhoque–Pereira R, Endres CM, Frazzon J, Prichula J, Frazzon–Guedes AP. Resistant enterococci isolated from raw sheep’s milk and cheeses from South region of Brazil. Cienc. Rural [Internet]. 2023; 53(10):e20220288. doi: https://doi.org/px3v DOI: https://doi.org/10.1590/0103-8478cr20220288
Freitas AR, Novais C, Duarte B, Pereira AP, Coque TM, Peixe L. High rates of colonisation by ampicillin–resistant enterococci in residents of long–term care facilities in Porto, Portugal. Int. J. Antimicrob. Agents [Internet]. 2018; 51(3):503-507. doi: https://doi.org/gc7tx2 DOI: https://doi.org/10.1016/j.ijantimicag.2017.11.007
Freitas AR, Tedim AP, Francia MV, Jensen LB, Novais C, Peixe L, Sánchez–Valenzuela A, Sundsfjord A, Hegstad K, Werner G, Sadowy E, Hammerum AM, Garcia–Migura L, Willems RJ, Baquero F, Coque TM. Multilevel population genetic analysis of vanA and vanB Enterococcus faecium causing nosocomial outbreaks in 27 countries (1986-2012). J. Antimicrob. Chemother. [Internet]. 2016; 71(12):3351-3366. doi: https://doi.org/gbpcnh DOI: https://doi.org/10.1093/jac/dkw312
Bortolaia V, Espinosa–Gongora C, Guardabassi L. Human health risks associated with antimicrobial–resistant enterococci and Staphylococcus aureus on poultry meat. Clin. Microbiol. Infect. [Internet]. 2016; 22(2):130-140. doi: https://doi.org/f8r93w DOI: https://doi.org/10.1016/j.cmi.2015.12.003
Joste V, Gydé E, Toullec L, Courboulès C, Talb Y, Riverain–Gillet E, Pangon B, Amara M. Enterococcus faecium and ampicillin susceptibility determination: overestimation of resistance with disk diffusion method using 2 micrograms of ampicillin?. J. Clin. Microbiol. [Internet]. 2019; 57(3):e01467-18. doi: https://doi.org/px3w DOI: https://doi.org/10.1128/JCM.01467-18
Hsieh SE, Hsu LL, Hsu WH, Chen CY, Chen HJ, Liao CT. Importance of amino acid alterations and expression of penicillin–binding protein 5 to ampicillin resistance of Enterococcus faecium in Taiwan. Int. J. Antimicrob. Agents [Internet]. 2006; 28(6):514-519. doi: https://doi.org/bs636z DOI: https://doi.org/10.1016/j.ijantimicag.2006.07.027
Aslantaş Ö. Molecular and phenotypic characterization of enterococci isolated from broiler flocks in Turkey. Trop. Anim. Health Prod. [Internet]. 2019; 51(5):1073-1082. doi: https://doi.org/px3x DOI: https://doi.org/10.1007/s11250-018-01784-z
Hunashal Y, Kumar GS, Choy MS, D’Andréa ÉD, Da Silva Santiago A, Schoenle MV, Desbonnet C, Arthur M, Rice LB, Page R, Peti W. Molecular basis of β–lactam antibiotic resistance of ESKAPE bacterium E. faecium penicillin binding protein PBP5. Nat. Commun. [Internet]. 2023; 14(1):4268. doi: https://doi.org/px3z DOI: https://doi.org/10.1038/s41467-023-39966-5
Hammad AM, Aly SS, Hassan HA, Abbas NH, Eltahan A, Khalifa E, Shimamoto T. Occurrence, phenotypic and molecular characteristics of vancomycin–resistant enterococci isolated from retail raw milk in Egypt. Foodborne Pathog. Dis. [Internet]. 2022; 19(3):192-198. doi: https://doi.org/px32 DOI: https://doi.org/10.1089/fpd.2021.0054
Kagkli DM, Vancanneyt M, Vandamme P, Hill C, Cogan TM. Contamination of milk by enterococci and coliforms from bovine faeces. J. Appl. Microbiol. [Internet]. 2007; 103(5):1393-1405. doi: https://doi.org/d9f5j5 DOI: https://doi.org/10.1111/j.1365-2672.2007.03338.x
Silvetti T, Morandi S, Brasca M. Does Enterococcus faecalis from traditional raw milk cheeses serve as a reservoir of antibiotic resistance and pathogenic traits?. Foodborne Pathog. Dis. [Internet]. 2019; 16(5):359-367. doi: https://doi.org/gmxmd3 DOI: https://doi.org/10.1089/fpd.2018.2542
Hammad AM, Hassan HA, Shimamoto T. Prevalence, antibiotic resistance and virulence of Enterococcus spp. in Egyptian fresh raw milk cheese. Food Control [Internet]. 2015; 50:815-820. doi: https://doi.org/px33 DOI: https://doi.org/10.1016/j.foodcont.2014.10.020
European Union (EU). Council Regulation No. 2821/98 of 17 December 1998 amending, as regards withdrawal of the authorization of certain antibiotics, Directive 70/524/EEC concerning additives in feeding stuffs. [Internet]. Official Journal of the European Communities. 1998 [cited Jan 14, 2023]. Available in: https://goo.su/Fxeg
Borgen K, Simonsen GS, Sundsfjord A, Wasteson Y, Olsvik Ø, Kruse H. Continuing high prevalence of vanA–type vancomycin–resistant enterococci on Norwegian poultry farms three years after avoparcin was banned. J. Appl. Microbiol. [Internet]. 2000; 89(3):478-485. doi: https://doi.org/dgh4xw DOI: https://doi.org/10.1046/j.1365-2672.2000.01137.x
Bortolaia V, Mander M, Jensen LB, Olsen JE, Guardabassi L. Persistence of vancomycin resistance in multiple clones of Enterococcus faecium isolated from Danish broilers 15 years after the ban of avoparcin. Antimicrob. Agents Chemother. [Internet]. 2015; 59(5):2926-2929. doi: https://doi.org/f7fv2v DOI: https://doi.org/10.1128/AAC.05072-14
Onaran B, Göncüoğlu M, Ormancı FSB. Antibiotic resistance profiles of vancomycin resistant enterococci in chicken meat samples. Ank. Univ. Vet. Fak. Derg. [Internet]. 2019; 66(4):331-336. doi: https://doi.org/px34 DOI: https://doi.org/10.33988/auvfd.451328
Aslantaş Ö. Investigation of faecal carriage of high–level gentamicin resistant Enterococci in dogs and cats. Isr. J. Vet. Med. [Internet]. 2022 [cited Jun 10, 2025]; 77:27-37. Available in: https://goo.su/QlqzVjW
Özdemir R, Tuncer Y. Detection of antibiotic resistance profiles and aminoglycoside–modifying enzyme (AME) genes in high– level aminoglycoside–resistant (HLAR) enterococci isolated from raw milk and traditional cheeses in Turkey. [Internet]. Mol. Biol. Rep. 2020; 47(3):1703-1712. doi: https://doi.org/px35 DOI: https://doi.org/10.1007/s11033-020-05262-4
Nasiri M, Hanifian S. Enterococcus faecalis and Enterococcus faecium in pasteurized milk: Prevalence, genotyping, and characterization of virulence traits. LWT–Food Sci. Technol. [Internet]. 2022; 153:112452. doi: https://doi.org/px36 DOI: https://doi.org/10.1016/j.lwt.2021.112452
Yalçın M, Tuncer Y. Determination of the antibiotic resistance profiles of high–level aminoglycoside–resistant enterococci isolated from broiler meat. Gıda. [Internet]. 2021; 46(4):803-816. doi: https://doi.org/px37 DOI: https://doi.org/10.15237/gida.GD21063
Martinez–Laorden A, Arraiz–Fernandez C, Gonzalez–Fandos E. Identification and characterisation of antimicrobial resistance of Enterococcus spp. isolated from pork and poultry meat. Int. J. Food Sci. [Internet]. 2023; 58(8):4455-4463. doi: https://doi.org/px38 DOI: https://doi.org/10.1111/ijfs.16562
Arias CA, Contreras GA, Murray BE. Management of multidrug– resistant enterococcal infections. Clin. Microbiol. Infect. [Internet]. 2010; 16(6):555-562. https://doi.org/dp836z DOI: https://doi.org/10.1111/j.1469-0691.2010.03214.x
Boccella M, Santella B, Pagliano P, De Filippis A, Casolaro V, Galdiero M, Borrelli A, Capunzo M, Boccia G, Franci G. Prevalence and antimicrobial resistance of Enterococcus species: A retrospective cohort study in Italy. Antibiotics [Internet]. 2021; 10(12):1552. doi: https://doi.org/gppfvg DOI: https://doi.org/10.3390/antibiotics10121552
de Jong A, Simjee S, Garch FE, Moyaert H, Rose M, Youala M, Dry M. Antimicrobial susceptibility of enterococci recovered from healthy cattle, pigs, and chickens in nine EU countries (EASSA Study) to critically important antibiotics. Vet. Microbiol. [Internet]. 2018; 216:168-175. doi: https://doi.org/gdbb74 DOI: https://doi.org/10.1016/j.vetmic.2018.02.010
Jamet E, Akary E, Poisson MA, Chamba JF, Bertrand X, Serror P. Prevalence and characterization of antibiotic–resistant Enterococcus faecalis in French cheeses. Food Microbiol. [Internet]. 2012; 31(2):191-198. doi: https://doi.org/p8zb DOI: https://doi.org/10.1016/j.fm.2012.03.009
Gołaś–Prądzyńska M, Rola JG. Occurrence and antimicrobial resistance of enterococci isolated from goat’s milk. J. Vet. Res. [Internet]. 2021; 65(4):449-455. doi: https://doi.org/gq8xkc DOI: https://doi.org/10.2478/jvetres-2021-0071
World Health Organization (WHO). Critically important antimicrobials for human medicine 6th. revision. WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR) [Internet]. Geneva (Switzerland): World Health Organization; 2019 [cited Jun 15, 2024]. Available in: https://goo.su/kYTnb
Biswas PP, Dey S, Adhikari L, Sen A. Virulence markers of vancomycin–resistant enterococci isolated from infected and colonized patients. J. Glob. Infect. Dis. [Internet]. 2014; 6(4):157-163. doi: https://doi.org/px4b DOI: https://doi.org/10.4103/0974-777X.145242
Paschoalini BR, Nuñez KVM, Maffei JT, Langoni H, Guimarães FF, Gebara C, Freitas NE, Dos Santos MV, Fidelis CE, Kappes R, Gonçalves MC, Silva NCC. The emergence of antimicrobial resistance and virulence characteristics in Enterococcus species isolated from bovine milk. Antibiotics [Internet]. 2023; 12(8):1243. doi: https://doi.org/px4c DOI: https://doi.org/10.3390/antibiotics12081243
Eaton TJ, Gasson MJ. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. [Internet]. 2001; 67(4):1628-1635. doi: https://doi.org/dsxdgz DOI: https://doi.org/10.1128/AEM.67.4.1628-1635.2001
Heikens E, van Schaik W, Leavis HL, Bonten MJ, Willems RJ. Identification of a novel genomic island specific to hospital– acquired clonal complex 17 Enterococcus faecium isolates. Appl. Environ. Microbiol. [Internet]. 2008; 74(22):7094-7097. doi: https://doi.org/b27g5b DOI: https://doi.org/10.1128/AEM.01378-08
Khan H, Flint S, Yu PL. Enterocins in food preservation. Int. J. Food Microbiol. [Internet]. 2010; 141(1-2):1-10. doi: https://doi.org/dqkxc7 DOI: https://doi.org/10.1016/j.ijfoodmicro.2010.03.005