Comparación de parámetros bioquímicos séricos en cabras de mohair de color Siirt con cetosis y sanas durante la lactancia temprana para identificar posibles biomarcadores de cetosis de lactancia
Resumen
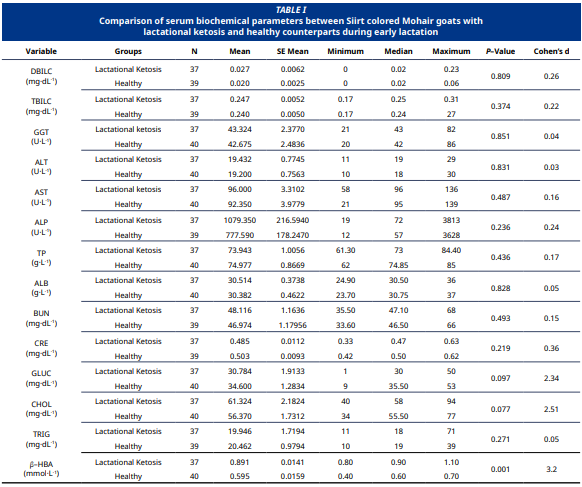
Este estudio tuvo como objetivo comparar los parámetros bioquímicos séricos entre cabras mohair de color Siirt cetósicas y sanas durante la lactancia temprana, con el fin de diagnosticar la cetosis subclínica de la lactancia e identificar posibles biomarcadores. Se evaluaron 77 cabras hembras, entre 2 y 5 años de edad y dentro de los 30 días posparto, bajo condiciones de manejo similares. Los animales se clasificaron en dos grupos según los niveles séricos de ácido β–hidroxibutírico: cetosis subclínica de la lactancia (n = 37) y controles sanos (n = 40). Los niveles de ácido β–hidroxibutírico fueron significativamente más altos en el grupo con cetosis (0,891 ± 0,0141 mmol·L-1) en comparación con el grupo control (0,595 ± 0,0159 mmol·L-1, P<0,001), lo que confirma su valor diagnóstico. Los demás parámetros séricos no mostraron diferencias significativas entre los grupos (P>0,05). Por otro lado, el análisis del tamaño del efecto reveló que el nivel de glucosa disminuyó en las cabras con cetosis subclínica de la lactancia, mientras que el colesterol aumentó (tamaño del efecto grande). El estudio concluyó que la mayoría de los indicadores hepáticos y metabólicos se mantuvieron dentro de los rangos normales, mientras que los niveles de glucosa y colesterol cambiaron debido a la cetosis subclínica de la lactancia. Estos datos indican que la alimentación basada en pastoreo bajo condiciones extensivas puede ser insuficiente para satisfacer los requerimientos energéticos de las cabras durante la lactancia, y enfatizan la importancia de la detección bioquímica temprano durante la lactancia para el manejo eficaz de los trastornos metabólicos en esta etapa inicial en las cabras. Por lo tanto, se recomienda realizar estudios adicionales para ampliar el conocimiento sobre el efecto de la cetosis de la lactancia en los cambios metabólicos en cabras.
Descargas
Citas
Thornton PK. Livestock production: recent trends, future prospects. Philos. Trans. R. Soc. B. Biol. Sci. [Internet]. 2010; 365(1554):2853-2867. doi: https://doi.org/b63ght DOI: https://doi.org/10.1098/rstb.2010.0134
Martens H. Increasing milk yield and negative energy balance: A gordian knot for dairy cows? Animals [Internet]. 2023; 13(19):3097. doi: https://doi.org/g926m8 DOI: https://doi.org/10.3390/ani13193097
Huang Y, Wen J, Kong Y, Zhao C, Liu S, Liu Y, Li L, Yang J, Zhu X, Zhao B, Cao B, Wang J. Oxidative status in dairy goats: periparturient variation and changes in subclinical hyperketonemia and hypocalcemia. BMC Vet. Res. [Internet]. 2021; 17(1):238. doi: https://doi.org/p655 DOI: https://doi.org/10.1186/s12917-021-02947-1
Chandu V, Kumari MK, Srija MSS, Naik KR, Srinivas G, Theja GK. Pregnancy toxaemia in goats: A review. Sci. World J. [Internet]. 2024; 4(9):3669-3675.doi: https://doi.org/p656
Simões J, Gutiérrez C. Nutritional and metabolic disorders in dairy goats. In: Simões J, Gutiérrez C, editors. Sustainable goat production in adverse environments, Vol. I. Vila Real, Portugal: Springer; 2018 [Cited Jun. 16, 2025]; p. 177-194. Available in: https://goo.su/u2732O DOI: https://doi.org/10.1007/978-3-319-71855-2_11
Wisnieski L, Norby B, Pierce S, Becker T, Gandy J, Sordillo L. Predictive models for early lactation diseases in transition dairy cattle at dry–off. Prev. Vet. Med. [Internet]. 2019; 163:68-78. doi: https://doi.org/p66b DOI: https://doi.org/10.1016/j.prevetmed.2018.12.014
Marutsova V, Binev R. Changes in blood enzyme activities and some liver parameters in goats with subclinical ketosis. Bulg. J. Vet. Med. [Internet]. 2020; 23(1):70-79. doi: https://doi.org/g5ggcp DOI: https://doi.org/10.15547/bjvm.2175
Kayri V, Irmak M, İrak K, Çelik ÖY. Comparison of some blood parameters in female romanov and hamdani sheep housed under the same nutritional conditions. Van. Vet. J. [Internet]. 2025; 36(1):8-13. doi: https://doi.org/p66h DOI: https://doi.org/10.36483/vanvetj.1562116
Paiano RB, Birgel DB, Bonilla J, Birgel Junior EH. Evaluation of biochemical profile of dairy cows with metabolic diseases in tropical conditions. Reprod. Domest. Anim. [Internet]. 2020;55(9):1219-1228. doi: https://doi.org/p66j DOI: https://doi.org/10.1111/rda.13768
Bani–Ismail Z, Al–Majali A, Amireh F, Al–Rawashdeh O. Metabolic profiles in goat does in late pregnancy with and without subclinical pregnancy toxemia. Vet. Clin. Pathol. [Internet]. 2008; 37(4):434-437. doi: https://doi.org/bxp8cn DOI: https://doi.org/10.1111/j.1939-165X.2008.00076.x
Oetzel GR. Monitoring and testing dairy herds for metabolic disease. Vet. Clin. North Am. Food Anim. Pract. [Internet]. 2004; 20(3):651-674. doi:https://doi.org/ccgxpc DOI: https://doi.org/10.1016/j.cvfa.2004.06.006
Ramin A, Asri–Rezai S, Macali S. Evaluation on serum glucose, BHB, urea and cortisol concentrations in pregnant ewes. Folia Vet. [Internet]. 2007 [Cited 31 Jun 2025]; 51(1):9-13. Available in: https://goo.su/ynU1U5i
Aly MA, Elshahawy II. Clinico–biochemical diagnosis of pregnancy toxemia in ewes with special reference to novel biomarkers. Alex. J. Vet. Sci. [Internet]. 2016; 48(2):96-102. doi: https://doi.org/g5rcqr DOI: https://doi.org/10.5455/ajvs.215993
Duffield T. Subclinical ketosis in lactating dairy cattle. Vet. Clin. North Am. Food Anim. Pract. [Internet]. 2000; 16(2):231-253, doi: https://doi.org/gj3dsq DOI: https://doi.org/10.1016/S0749-0720(15)30103-1
Tams TR. Handbook of Small Animal Gastroenterology. 2nd Ed. St. Louis (USA):Saunders; 2003.
Constable PD, Hinchcliff KW, Done SH, Grünberg W. Veterinary medicine: A textbook of the diseases of cattle, horses, sheep, pigs and goats. 11th Ed. Philadelphia (USA):Elsevier; 2016.
Đoković R, Ilić Z, Kurćubić V, Petrović M, Cincović M, Petrović MP, Caro–Perović V. Diagnosis of subclinical ketosis in dairy cows. Biotechnol. Anim. Husb. [Internet]. 2019; 35(2):111-125. doi: https://doi.org/p67c DOI: https://doi.org/10.2298/BAH1902111D
Shin E, Jeong JK, Choi IS, Kang HG, Hur TY, Jung YH, Kim IH. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows. Theriogenology. [Internet]. 2015; 84(2):252-260. doi: https://doi.org/gj3djc DOI: https://doi.org/10.1016/j.theriogenology.2015.03.014
Turgut AO, Koca D, Ünver A. Comparison of blood BHBA measurement devices for diagnosis of subclinical pregnancy toxaemia in sheep: A field study. Reprod. Domest. Anim. [Internet]. 2024; 59(5):e14589. doi: https://doi.org/p67g DOI: https://doi.org/10.1111/rda.14589
Hildebrand C, Hollenbach J, Seeger B, Pfarrer C. Beta– Hydroxybutyrate effects on bovine caruncular epithelial cells: A model for investigating the peri–implantation period disruption in ketotic dairy cows. Animals [Internet]. 2023; 13(18):2950. doi: https://doi.org/p67h DOI: https://doi.org/10.3390/ani13182950
Irmak M, Kayri V, Tufan T, Coşkun D, Özcan C, Çelik Ö, Denli M. The effect of β–carotene and vitamin E on metabolic profiles in nutritionally flushed sheep. S. Afr. J. Anim. [Internet]. 2022; 52(6):867-872. doi: https://doi.org/p67j DOI: https://doi.org/10.4314/sajas.v52i6.12
Turgut AO, Kucuk M, Irmak M, Ozcan C, Koca D, Gulendag E, Onen MF, Dogan R, Unver A, Keskin IH. Subclinical pregnancy toxemia affects blood parameters of ewes and impairs postnatal growth and development of lambs. Vet. Med. Sci. [Internet]. 2025; 11(3):e70259. doi: https://doi.org/p67k DOI: https://doi.org/10.1002/vms3.70259
Vasava PR, Jani RG, Goswami HV, Rathwa SD, Tandel FB. Studies on clinical signs and biochemical alteration in pregnancy toxemic goats. Vet. World [Internet]. 2016; 9(8):869-874. doi: https://doi.org/g5ggbz DOI: https://doi.org/10.14202/vetworld.2016.869-874
Zobel G, Leslie K, Weary DM, von Keyserlingk MA. Ketonemia in dairy goats: effect of dry period length and effect on lying behavior. J. Dairy Sci. [Internet]. 2015; 98(9):6128-6138. doi: https://doi.org/f7pfk8 DOI: https://doi.org/10.3168/jds.2014-9136
Yadav SN, Kalita D, Phukan A, Das B, Dutta T, Mahato G, Tamuly S, Barman D, Bharali K. A comparative therapeutic study on subclinical ketosis of goat. J. Entomol. Zool. Stud. [Internet]. 2018; [Cited Jun. 31, 2025]; 6(3):673-676. Available in: https://goo.su/6Srvn
González FD, Muiño R, Pereira V, Campos R, Benedito JL. Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high–yielding dairy cows. J. Dairy Sci. [Internet]. 2011; 12(3):251-255. doi: https://doi.org/dd2sp2 DOI: https://doi.org/10.4142/jvs.2011.12.3.251
Overton T, Waldron M. Nutritional management of transition dairy cows: strategies to optimize metabolic health. J. Dairy Sci. [Internet]. 2004; 87:E105-E119. doi: https://doi.org/dcgtn5 DOI: https://doi.org/10.3168/jds.S0022-0302(04)70066-1
Tufarelli V, Puvača N, Glamočić D, Pugliese G, Colonna MA. The most important metabolic diseases in dairy cattle during the transition period. Animals [Internet]. 2024; 14(5):816. doi: https://doi.org/p67m DOI: https://doi.org/10.3390/ani14050816
Bertoni G, Trevisi E. Use of the liver activity index and other metabolic variables in the assessment of metabolic health in dairy herds. Vet. Clin. North Am. Food Anim. Pract. [Internet]. 2013; 29(2):413-431. doi: https://doi.org/g7vhp4 DOI: https://doi.org/10.1016/j.cvfa.2013.04.004
Iluz–Freundlich D, Zhang M, Uhanova J, Minuk GY. The relative expression of hepatocellular and cholestatic liver enzymes in adult patients with liver disease. Ann. Hepatol. [Internet]. 2020; 19(2):204-208. doi: https://doi.org/g9j74v DOI: https://doi.org/10.1016/j.aohep.2019.08.004
Liu S, Kong Y, Wen J, Huang Y, Liu Y, Zhu X, Zhao B, Cao B, Wang J. Surrogate indexes of insulin resistance in dairy goats: Transitional variation in subclinical hyperketonemia. Vet. Sci. [Internet]. 2021; 8(6):102. doi: https://doi.org/gkf2vq DOI: https://doi.org/10.3390/vetsci8060102
Huang Y, Kong Y, Shen B, Li B, Loor JJ, Tan P, Wei B, Mei L, Zhang Z, Zhao C, Zhu X, Qi S, Wang J. Untargeted metabolomics and lipidomics to assess plasma metabolite changes in dairy goats with subclinical hyperketonemia. J. Dairy Sci. [Internet]. 2023; 106(5):3692-3705. doi: https://doi.org/p67q DOI: https://doi.org/10.3168/jds.2022-22812
Şahal M, Çolakoğlu EÇ, Alihosseini H. Ketozis ve yağlı karaciğer sendromunun tedavisinde güncel yaklaşımlar ve tedavideki başarısızlığın nedenleri [Ketosis and fatty liver syndrome: current insights and causes of failure in treatment]. Turkiye Klinikleri J. Vet. Sci. [Internet]. 2011; [Cited July 31, 2025]; 2(2):140-150. Turkish. Available in: https://goo.su/k0wf0
Iqbal R, Beigh SA, Mir AQ, Shaheen M, Hussain SA, Nisar M, Dar AA. Evaluation of metabolic and oxidative profile in ovine pregnancy toxemia and to determine their association with diagnosis and prognosis of disease. Trop. Anim. Health Prod. [Internet]. 2022; 54(6):338. doi: https://doi.org/g5ggb8 DOI: https://doi.org/10.1007/s11250-022-03339-9
Kelay A, Assefa A. Causes, control and prevention methods of pregnancy toxemia in ewe: A review. J. Life Sci. Biomed. [Internet]. 2018; [Cited Jun 31, 2025]; 8(4):69-76. Available in: https://goo.su/QdgEzL
Simões P, Bexiga R, Lamas L, Lima M. Pregnancy toxaemia in small ruminants. In: Freitas Duarte A, Lopes da Costa L, editors. Advances in animal health, medicine and production. Lisbon (Portugal): Springer; 2020; p. 541-556. DOI: https://doi.org/10.1007/978-3-030-61981-7_30